- Scientific name: Lithobates pipiens
- Species of Greatest Conservation Need (MA State Wildlife Action Plan)

Description
Northern leopard frog is a medium-sized, spotted frog with variable coloration. Spots are always dark (black or brown), rounded (circular to elliptical), encircled by a thin, light-colored (whitish to neon green) halo, and distributed irregularly over the back and sides of the body. The dorsal base color of northern leopard frog can vary from a dull tan to a brilliant green. The undersides are white and unmarked. Length (from snout to vent) is 5-9 cm (2-3.5 inches), and females are typically larger than males (especially when gravid). Tadpoles have olive to brownish-colored bodies with whitish undersides and dark-speckled tails.
Similar species
Pickerel frog (Lithobates palustris) is commonly confused with northern leopard frog. However, the spots of pickerel frog are typically dark brown bordered by a thin line of black, rectangular in shape, and distributed in two parallel rows down the back (as well as a single prominent row along each side). The dorsal base color of pickerel frog is always brownish (never green), and the inner thighs of adults and older juveniles are colored bright yellow to orange.
Life cycle and behavior
Northern leopard frogs spend winters in shallow, excavated pits at the bottoms of ponds and other permanent wetlands where they lie dormant until ice thaws and waters begin to warm in mid- to late March. In Massachusetts, the breeding season usually commences in early to mid-April, whereupon the frogs become active and move into shallower waters with emergent vegetation or migrate to breeding sites. When overland movements are necessary to reach breeding sites, they tend to occur during warm (>4.4 °C; >40 °F), nocturnal rains, coincident with breeding migrations of mole salamanders (Ambystoma spp.) and wood frogs (Lithobates sylvaticus). Once daytime temperatures reach 10 °C (50 °F) and nighttime temperatures hold at 7-10 °C (mid- to high 40s), male northern leopard frogs begin calling to attract mates.
The call of the northern leopard frog is a two-parted sequence. It begins with a slow, guttural crescendo of staccato “knocking” notes, followed by a series of several short, nasal grunts. Calling activity is generally slow, truncated, and sporadic during cool temperatures and at the beginning of the breeding period. However, once warm, sunny days and warm nights arrive, many males erupt into a full chorus of calling for several days to a week. The overlapping calls of dozens of males combine to produce a resonant, ethereal chorus that cannot be mistaken for any other animal in our area.
Females, already carrying eggs, evaluate the males and select their mates. The male grasps the female from above with his forelimbs tucked securely beneath hers, holds her in place, and fertilizes her eggs as she releases them into the water (a behavior termed amplexus). The eggs are deposited in a mass that resembles a flattened sphere and contains up to 6,000 individual eggs.
When gravid, female northern leopard frogs may be distinguished easily from males merely by their girth.
Eggs hatch in several weeks, whereupon the frog tadpoles remain in the water for 2-3 months. During that time, the tadpoles feed on algae, plant matter, organic debris, and possibly small animal matter filtered from the water or scraped from surfaces. As the tadpoles grow, they develop limbs and metamorphose into small, juvenile frogs in July or August.
Recently metamorphosed frogs usually congregate near the margins of their natal wetlands, feeding along banks or under the cover of floodplain vegetation. During late summer and early fall, adults and sub-adults return from upland fields, meadows, and forested swamps to their overwintering sites where they, too, will forage along the margins of the wetlands until cold temperatures force them into deep water for the winter.
The diet of juvenile and adult northern leopard frogs consists primarily of invertebrates, with crickets and grasshoppers being reported favorites. Juveniles are believed to mature in 2 years, with total life expectancy seldom exceeding 4 or 5 years in the wild.
Distribution and abundance
Northern leopard frog occurs across most of northern North America, ranging from southern Quebec west to southern Alberta and eastern portions of Washington, Oregon, and California. The range extends across New England, New York, the Great Lakes States and the Upper Midwest, south to Arizona and New Mexico. Disjunct populations occur in Labrador and the southern Northwest Territories.
Within Massachusetts, populations of northern leopard frog are scattered among portions of at least 7 counties: Berkshire, Essex, Hampshire, Middlesex, Norfolk, Plymouth, and Worcester. Observation data suggest the species is distributed sparsely, but it is abundant locally. As of February 2025, approximately 23 local populations among 27 towns had been documented and reported to the Massachusetts Natural Heritage & Endangered Species Program since 2000. Other populations undoubtedly occur among additional towns. Prior records indicate the species once occupied parts of Bristol County and Hampden County, but survey results and an absence of incidental observation reports since that time suggest local populations have either declined in or been extirpated from those areas.
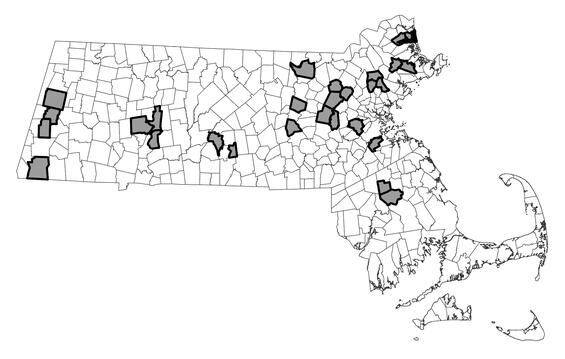
Distribution in Massachusetts.
2000-2024
Based on records in the Natural Heritage Database and on additional NHESP project data.
Population status
Although northern leopard frog is secure globally, it is deserving of conservation attention in Massachusetts. The sparse distribution of the species in Massachusetts, combined with an apparent contraction in its range both within the state and elsewhere in New England during the past several decades, has led to concerns about likely population declines. One complicating factor is that the species was once commonly acquired from biological supply companies for use in academic laboratories, and some scientists have suspected that released lab animals (or other releases) may have contributed to historic occurrences of the species on the Massachusetts landscape many decades ago. Accordingly, it is challenging to interpret observed range contractions. The conservation status of northern leopard frog in our area remains uncertain.
Habitat
Northern leopard frog utilizes both aquatic and terrestrial habitat. The primary aquatic habitats are used for overwintering and breeding. Overwintering sites are typically cold, well-oxygenated water bodies and may include lakes, ponds, rivers, streams, and springs at or near breeding sites. Breeding sites usually consist of extensive marshes and shrub swamps bordering streams, rivers, lakes, and ponds. Those wetland systems are often circumneutral to calcareous and contain much emergent vegetation (e.g., Typha spp., Cephalanthus occidentalis). In the surrounding landscape, northern leopard frogs may breed in shallow ponds and isolated shrub swamps with semipermanent to permanent hydroperiods. The species is also found in seasonal ponds, which are presumably important stopover habitats for hydration during terrestrial movements (though eggs may be deposited in them occasionally).
Healthy habitats are vital for supporting native wildlife and plants. Explore habitats and learn about conservation and restoration in Massachusetts.
Marshes bordering small rivers host some of the largest populations of northern leopard frog in Massachusetts.
During late spring, many adult and subadult northern leopard frogs move into upland fields, grasslands, and wet meadows to feed through late summer or early fall. Open shrublands and regenerating clearcuts are also used. Some individuals may travel up to 3 km (1.8 mi) from permanent water bodies to reach preferred foraging areas.
As in some other amphibian species, local populations of northern leopard frog occur in varying complexity on the landscape. At one end of the spectrum, a resident population is restricted to a single breeding wetland within an isolated patch of upland habitat (i.e., there is no emigration to or immigration from other populations). However, at the other end of the spectrum, multiple subpopulations occur as an interactive network (or metapopulation) across an extensive area of upland habitat containing a multitude of breeding sites. Individuals from a given subpopulation are free to disperse away from its central breeding area and bordering uplands, emigrating to a different subpopulation and its breeding site, recolonizing the area of a formerly extirpated subpopulation, or pioneering an entirely new subpopulation. Hence, the land (and water) areas between and among active and prospective breeding sites provide important dispersal habitat for northern leopard frogs, allowing individuals to move among subpopulations. Watercourses, wet meadows, and swamp forest are preferred types of dispersal habitat. The most critical aspect of suitable dispersal habitat is that it does not contain major barriers to frog movement (e.g., vast dry areas, extensive vertical structures, roads with high nightly traffic volume). Dispersal habitat is key to the maintenance of a metapopulation and the ecological benefits that metapopulation dynamics confer to long-term population viability in the face of environmental and other stressors. Where appropriate habitat mosaics are contiguous, a robust metapopulation may span miles.
Threats
Primary threats to northern leopard frog in Massachusetts are habitat loss, habitat degradation, habitat isolation, climate change, anthropogenic mortality, and disease. These threats may act alone or in combination to cause direct, indirect, and/or cumulative impacts to a given population.
Habitat loss
The most common type of habitat loss is residential, commercial, industrial, or mining development in upland fields, meadows, and shrublands. However, filling of vernal pools may also result in loss of valuable stopover habitat in landscapes where individuals disperse long distances between population centers.
Habitat degradation
Habitat degradation typically occurs when development and roads fragment habitat, creating gaps or obstacles that present increased risks of frog desiccation and predation, or increase the travel distances and times needed for individuals to reach destinations (i.e., when frogs attempt to go around rather than through a gap or obstacle). Runoff from roads, parking lots, lawns, crop fields, and other areas introduces chemicals (e.g., petroleum, deicing salts, fertilizers, pesticides) and/or sediments to breeding wetlands, potentially disrupting or inhibiting embryonic development or tadpole growth and survival. Environmental acidification threatens northern leopard frog reproduction in aquatic habitats. Noise pollution from increasing road densities and traffic volumes may alter frog calling behavior in ways that either impair breeding activity or result in certain tradeoffs that could conceivably reduce reproductive fitness. Field and meadow habitats are degraded when non-native, invasive plant species – especially the thorny and shade-producing multiflora rose (Rosa multiflora) – are allowed to colonize and take over a site. Direct dumping of refuse (tires, batteries, oil filters, paint cans, etc.) into wetlands is another common form of habitat degradation.
Habitat isolation
When habitat loss and fragmentation result in local populations of northern leopard frog becoming isolated (i.e., the potential for immigration is eliminated), they lose the benefits of metapopulation dynamics. In particular, hypothetical rescue effect – the principle of dispersing individuals recolonizing the site of an extirpated subpopulation, thereby rescuing the subpopulation and bolstering the broader metapopulation – becomes an impossibility. Furthermore, population isolation can lead to declines in genetic diversity within the population. Metapopulations, due to the connectedness and genetic diversity of their subpopulations, are considered more resistant to environmental change and more resilient in response to local extinctions. Therefore, metapopulations are generally more viable than isolated populations over the long term. Habitat isolation might be a contributing factor in the apparent disappearances of northern leopard frog from some parts of its New England range during the past several decades.
Climate change
A 2024 synthesis of climate data, climate modeling, and climate-related research indicates that temperature, total annual precipitation, and frequency of heavy precipitation events are trending upward in the northeastern United States and are expected to continue to do so. A warming and wetting trend might intuitively suggest potential benefits to amphibians, though that might not be true for northern species, like northern leopard frog, whose populations in Massachusetts are closer to the southern portion of the species’ geographic range in eastern North America. The timing, frequency, and intensity of precipitation events are important considerations in evaluating the potential impacts of climate change.
Climate data indicate that the Northeast is experiencing wetter summers and falls and drier winters and springs. Such a shift in precipitation patterns threatens the viability of some northern leopard frog breeding habitats, as wetlands may begin the breeding period with lower water volumes and – with continued seasonal drought – draw down to critical levels. Lower water volumes may cause increased tadpole densities and competition for food, thereby slowing growth rates and extending the amount of time needed to reach metamorphosis. If the waters of a marsh or swamp bordering a stream or river draw down excessively during spring, tadpoles must follow the receding waters and eventually may be forced to move into the main channel where they are more vulnerable to predation by fish. If such a scenario increases in frequency over time, reduced productivity and declines in population size could be expected to follow.
Erratic precipitation patterns might also negatively impact the hydrological stability of wet meadows, a preferred spring and summer habitat. Reduced stability of the habitat could result in extended periods of dryness, overgrowth of vegetation, and sudden flooding events, thereby altering habitat function and quality for northern leopard frogs.
Anthropogenic mortality
Like other amphibians, northern leopard frog is susceptible to direct mortality at the hands of Homo sapiens. The primary source of anthropogenic mortality is likely automobiles. Despite being quicker than the average amphibian and capable of sizeable leaps, northern leopard frogs are no match for a busy road during cool weather. Some individuals need to cross over roads to reach preferred breeding areas during early spring, when cold temperatures limit frog mobility and responsiveness. Even later in the spring, when many individuals must cross over roads to reach fields and meadows, busy roads present an unnatural form of predation. Where populations are surrounded by development and little suitable upland habitat exists, most frogs attempting to escape the habitat island are undoubtedly killed, limiting the size of the adult population. Increasing traffic volumes and mortality rates over time are likely to cause population declines and, in combination with other threats, local extinctions.
At a smaller scale, adult and subadult northern leopard frogs are vulnerable to hayfield mowers, especially where hayfields occur near wetlands. This is an understudied threat in New England, but a study of 59 radio-tracked northern leopard frogs in a Minnesota population observed that at least 13% of study animals were killed by mowers over a 2-year period.
Disease
Infectious disease has been a significant contributor to global amphibian declines over the past several decades, but impacts in Massachusetts and other parts of New England are not entirely clear.
The “amphibian chytrid” Batrachochytrium dendrobatidis (Bd), which is believed to have originated in Asia and spread globally via the pet trade, is a fungal pathogen now prevalent throughout Massachusetts. It is widely blamed for amphibian population declines and extinctions in some parts of the world. Northern leopard frog appears to be resistant to severe Bd infection and is not known to incur population declines from Bd exposure in the Northeast. Peptides in the skin appear to be protective, though some research suggests exposure can reduce growth and feeding rates in juvenile northern leopard frogs, resulting in reduced body size and, therefore, potentially reduced fecundity and lifetime reproductive potential.
Another major amphibian disease of concern is the group of viruses known as ranaviruses (family Iridoviridae). Like Bd, ranaviruses are believed to have contributed significantly to global amphibian declines. Ranaviruses were first isolated from northern leopard frogs in the 1960s. They are established in Massachusetts and are one of the first suspects when people encounter mass mortality of mature amphibian larvae. Multiple common amphibian species in Massachusetts are carriers and effective spreaders of ranaviruses, and so introductions and outbreaks at northern leopard frog breeding sites are probably unavoidable. Northern leopard frog is considered susceptible to ranaviruses, with both infection and mortality observed in the wild. Sustained or repeated outbreaks at a given breeding site could threaten the local population of northern leopard frog with reduced productivity and, therefore, long-term decline.
Of particular concern with infectious amphibian disease is the potential for introduction and spread of novel strains via the commercial pet trade. Release of unwanted pets is a potential pathway for foreign strains of Bd or ranaviruses to enter the Massachusetts environment and infect native amphibian populations, many of which are already dealing with other stressors.
Conservation
Some prospective conservation measures for northern leopard frog in Massachusetts may be grouped into three general categories: inventory and monitoring, management, and research. MassWildlife’s Natural Heritage & Endangered Species Program (NHESP) is the state authority for coordinating and implementing such measures, often in collaboration with other agencies, academic institutions, land trusts, municipal conservation departments, private-sector herpetologists, and others. The NHESP also facilitates participation by the general public.
Inventory and monitoring
Inventory surveys are essential to discovering undocumented populations or subpopulations of northern leopard frog on the Massachusetts landscape and to evaluating the relative robustness of each. Knowledge about population abundance and distribution is prerequisite to understanding the conservation status of the species and to making informed decisions about how and where to invest scarce conservation resources for implementation of management strategies. As part of a regional assessment of the recently described species Atlantic Coast leopard frog (Lithobates kauffeldi), the NHESP and its collaborators documented incidental observations of northern leopard frog at a number of sites across the state during 2013-2015 and collected genetic samples from a subset of those populations. Northern leopard frog has been relatively understudied in Massachusetts since, and a new assessment is warranted.
Periodic monitoring surveys are necessary to stay informed about population statuses over time. These surveys provide insight about the effectiveness of certain management strategies, and they serve to detect site-specific threats or signs of population decline or extirpation. Standard measures of water chemistry – especially pH and dissolved oxygen – would be useful in a northern leopard frog monitoring program.
Management
At a local scale, northern leopard frog sites should be managed to develop or maintain meadows and grasslands adjacent to confirmed and potential breeding wetlands. Such management should focus on sites where threats to frogs (e.g., chemical pollution, roads) are absent or minor. When possible, mowing of grasslands and wet meadows should be done on a rotational basis or in the fall, preferably after mid-October. Hydrological regimes of overwintering sites and breeding wetlands should not be altered in ways that reduce hydroperiod at critical times or permanently inundate floodplains. Riparian buffers should be established in agricultural areas where chemical applications and/or soil erosion occur.
At the landscape scale, habitat mosaics consisting of marshes, wet meadows, grasslands, and swamp forest should be maintained to provide dispersal corridors and, therefore, allow for genetic exchange between among subpopulations and/or recolonization of formerly occupied habitat. Land acquisition and protection efforts for maintaining habitat connectivity should prioritize rural areas with low road and development densities. A land-protection strategy may best serve long-term persistence of local populations and preservation of metapopulation dynamics where northern leopard frogs occupy relatively large, connected areas containing suitable upland and wetland habitat mosaics. However, lands supporting small, peripheral, or isolated populations are also worth protecting for maintenance of genetic diversity at the state level.
Wet meadows and upland fields bordering marshes represent an ideal habitat configuration for northern leopard frogs in Massachusetts.
Stronger controls are needed to guard against the introduction and spread of amphibian pathogens and infectious disease. For example, possession of and commerce in amphibian species that are listed as Injurious Wildlife by the U.S. Fish and Wildlife Service due to disease concerns should be regulated at both the federal and state levels. In the natural environment, field biologists, researchers, anglers, and others that enter aquatic habitats should adopt and promote appropriate equipment-sanitation procedures between sites, especially when activities span wide geographic areas. A statewide wetland monitoring program that includes non-invasive sampling for pathogens (e.g., Bd, ranavirus) and surveillance for amphibian die-offs is needed.
Research
Research is necessary to help fill or improve upon knowledge gaps that might otherwise limit the effectiveness of current practices in conservation planning and management. There is also a need to measure known and suspected threats to northern leopard frog in Massachusetts so that perceived population declines might be better understood. Some general topics of conservation research interest include:
- Trends in wetland hydrological regimes, climate change, and frog breeding phenology;
- Prevalence of northern leopard frog mass mortality events caused by ranaviruses;
- Trends in environmental acidity at northern leopard frog breeding sites; and
- Identification of genetically distinct or unusual populations in Massachusetts at the state and regional level.

Scientists believe populations of northern leopard frog are in decline in some parts of New England, and so research to identify the most likely cause is needed.
Community participation
The general public is encouraged to participate in the conservation of northern leopard frog in several ways. For example, observations of northern leopard frog should be reported to the NHESP, as status assessment and potential land-protection efforts depend on knowing where local populations occur. Collection and submission of data for the certification of vernal pool habitat in the vicinity of northern leopard frog populations is a potentially beneficial action, as such wetlands are likely to be used at least occasionally by the species and will be afforded certain legal protections if certified. The Massachusetts community may also provide important information by reporting observations of mass amphibian mortality at vernal pools and other wetlands. All Massachusetts residents are urged to be mindful of the weather, plan ahead, and attempt to minimize the number of times they drive through rural areas near fields and marshes on rainy nights during spring and summer (i.e., when frogs are most likely to be on the move and attempting to cross over roadways).
Private landowners in areas of northern leopard frog habitat are encouraged to manage their properties in ways that minimize harm to a local population and maximize available habitat or microhabitat. Some general measures include minimizing landscaping, avoiding use of fertilizers and pesticides, and allowing lawns to overgrow. Accidental frog mortality during mowing of maintained fields or overgrown lawns can be minimized by delaying mowing until mid-fall. Avoid filling or regrading seasonally wet areas. If a property does include a landscaped water feature, encourage lush growth of knee-high grasses and other terrestrial plants alongside it, and avoid stocking it with fish or any non-native species. Making one’s property frog friendly is one of the best ways for residents to participate in conservation of northern leopard frog and other species.
References
Andrews, K.M., J.W. Gibbons, and D.M. Jochimsen. 2008. Ecological effects of roads on amphibians and reptiles: a literature review. Pages 121–143 in J. C. Mitchell, R. E. Jung Brown, and B. Bartholomew, editors. Urban Herpetology. Society for the Study of Amphibians and Reptiles, Salt Lake City, Utah, USA.
Baker, N.J., B.A. Bancroft, and T.S. Garcia. 2013. A meta-analysis of the effects of pesticides and fertilizers on survival and growth of amphibians. Science of the Total Environment 449:150-156.
Bartelt, P.E. and R.W. Klaver. 2017. Response of anurans to wetland restoration on a Midwestern agricultural landscape. Journal of Herpetology 51:504–514.
Blomquist, S.M., and M.L. Hunter, Jr. 2009. A multi-scale assessment of habitat selection and movement patterns by Northern Leopard Frogs (Lithobates [Rana] pipiens) in a managed forest. Herpetological Conservation and Biology 4:142–160.
Bouchard, J., A.T. Ford, F.E. Eigenbrod, and L. Fahrig. 2009. Behavioral responses of Northern Leopard Frogs (Rana pipiens) to roads and traffic: implications for population persistence. Ecology and Society 14(2):23. <http://www.ecologyandsociety.org/vol14/iss2/art23/>
Burgett, A.A., C.D. Wright, G.R. Smith, D.T. Fortune, and S.L. Johnson. 2007. Impact of ammonium nitrate on wood frog (Rana sylvatica) tadpoles: effects on survivorship and behavior. Herpetological Conservation and Biology 2:29–34.
Croteau, M.C., N. Hogan, J.C. Gibson, D. Lean, and V.L. Trudeau. 2008. Toxicological threats to amphibians and reptiles in urban environments. Pages 197–209 in J. C. Mitchell, R. E. Jung Brown, and B. Bartholomew, editors. Urban Herpetology. Society for the Study of Amphibians and Reptiles, Salt Lake City, Utah, USA.
Cunnington, G.M., and L. Fahrig. 2010. Plasticity in the vocalizations of anurans in response to traffic noise. Acta Oecologica 36:463–470.
Dole, J.W. 1965. Summer movements of adult leopard frogs, Rana pipiens Schreber, in northern Michigan. Ecology 46:236–255.
Dole, J.W. 1967. Spring movements of adult leopard frogs, Rana pipiens, in northern Michigan. American Midland Naturalist 78:167–181.
Dole, J.W. 1971. Dispersal of recently metamorphosed leopard frogs, Rana pipiens. Copeia 1971:221–228.
Emery, A.R., A.H. Berst, and K. Kodaira. 1972. Under-ice observations of wintering sites of leopard frogs. Copeia 1972:123–126.
Fahrig, L., and T. Rytwinski. 2009. Effects of roads on animal abundance: an empirical review and synthesis. Ecology and Society 14(1):21. <http://www.ecologyandsociety.org/vol14/iss1/art21/>
Force, E.R. 1933. The age of the attainment of sexual maturity of the leopard frog Rana pipiens (Schreber) in northern Michigan. Copeia 1933:128–131.
Freda, J., and W.A. Dunson. 1985. Field and laboratory studies of ion balance and growth rates of ranid tadpoles chronically exposed to low pH. Copeia 1985:415–423.
Freda, J., and D.H. Taylor. 1992. Behavioral response of amphibian larvae to acid water. Journal of Herpetology26:429–433.
Gray, M.J. and V.G. Chinchar, editors. 2015. Ranaviruses: lethal pathogens of ectothermic vertebrates. Springer Open. <https://doi.org/10.1007/978-3-319-13755-1_4>
Gray, M.J., D.L. Miller, and J.T. Hoverman. 2009. Ecology and pathology of amphibian ranaviruses. Diseases of Aquatic Organisms 87:243–266.
Green, D.E., K.A. Converse, and A.K. Schrader. 2002. Epizootiology of sixty-four amphibian morbidity and mortality events in the USA, 1996–2001. Annals of the New York Academy of Sciences 969:323–339.
IUCN, Conservation International, and NatureServe. 2004. Global Amphibian Assessment. IUCN, Conservation International, and NatureServe, Washington, DC and Arlington, Virginia, USA. <http://explorer.natureserve.org>
Karns, D.R. 1992. Effects of acidic bog habitats on amphibian reproduction in a northern Minnesota peatland. Journal of Herpetology 26:401–412.
Karraker, N.E., and J.P. Gibbs. 2011. Road deicing salt irreversibly disrupts osmoregulation of salamander egg clutches. Environmental Pollution 159:833–855.
Kenney, L.P., and M.R. Burne. 2000. A Field Guide to the Animals of Vernal Pools. Massachusetts Natural Heritage & Endangered Species Program, Westborough, Massachusetts, and Vernal Pool Association, Reading, Massachusetts, USA.
Klemens, M.W. 1993. Amphibians and reptiles of Connecticut and adjacent regions. State Geological and Natural History Survey of Connecticut. Bulletin 112.
Klemens, M.W., H.J. Gruner, D.P. Quinn, and E.R. Davison. 2021. Conservation of amphibians and reptiles in Connecticut. Department of Energy and Environmental Protection, Hartford, Connecticut, USA.
Knowlton, G.F. 1944. Some insect foods of Rana pipiens. Copeia 1944:119.
Kolby, J.E. and P. Daszak. 2016. The emerging amphibian fungal disease, chytridiomycosis: a key example of the global phenomenon of wildlife emerging infectious diseases. Microbiology Spectrum 4(3): EI10-0004-2015. doi:10.1128/microbiolspec.EI10-0004-2015.
Knutson, M.G., J.H. Herner-Thogmartin, W.E. Thogmartin, J.M. Kapfer, and J.C. Nelson. 2018. Habitat selection, movement patterns, and hazards encountered by northern leopard frogs (Lithobates pipiens) in an agricultural landscape. Herpetological Conservation and Biology 13:113–130.
Lannoo, M.J. and R.M. Stiles. 2020. Uncovering shifting amphibian ecological relationships in a world of environmental change. Herpetologica 76:144–152.
Linzey, D.W. 1967. Food of the leopard frog, Rana pipiens, in central New York. Herpetologica 23:11–17.
Longcore, J.R., J.E. Longcore, A.P. Pessier, and W.A. Halteman. 2007. Chytridiomycosis widespread in anurans of northeastern United States. Journal of Wildlife Management 71:435–444.
Lovett, G.M., T.H. Tear, D.C. Evers, S.E.G. Findlay, B.J. Cosby, J.K. Dunscomb, C.T. Driscoll, and K.C. Weathers. 2009. Effects of air pollution on ecosystems and biological diversity in the eastern United States. Annals of the New York Academy of Sciences 1162:99–135.
Merrell, D.J. 1970. Migration and gene dispersal in Rana pipiens. American Zoologist 10:47–52.
McQuigg, J. L., K. Kissner, and M.D. Boone. 2023. Exposure to amphibian chytrid fungus alters terrestrial growth and feeding rate in metamorphic amphibians. Journal of Herpetology 57:36–41.
Pace, A.E. 1974. Systematic and biological studies of the leopard frogs (Rana pipiens complex) of the United States. Miscellaneous Publications of the University of Michigan Museum of Zoology 148:1–140.
Parris, K.M., M. Velik-Lord, and J.M.A. North. 2009. Frogs call at a higher pitch in traffic noise. Ecology and Society 14(1):25. <http://www.ecologyandsociety.org/vol14/iss1/art25/>
Pask, J.D., T.L. Cary, and L.A. Rollins-Smith. 2013. Skin peptides protect juvenile leopard frogs (Rana pipiens) against chytridiomycosis. Journal of Experimental Biology 216:2908–2916.
Picco, A.M., and J.P. Collins. 2008. Amphibian commerce as a likely source of pathogen pollution. Conservation Biology 22:1582–1589.
Pope, S.E., L. Fahrig, and H.G. Merriam. 2000. Landscape complementation and metapopulation effects on leopard frog populations. Ecology 81:2498–2508.
Raithel, C.J. 2019. Amphibians of Rhode Island. Rhode Island Division of Fish and Wildlife, West Kingston, Rhode Island, USA.
Richards-Hrdlicka, K.L., J.L. Richardson, and L. Mohabir. 2013. First survey for the amphibian chytrid fungus Batrachochytrium dendrobatidis in Connecticut (USA) finds widespread prevalence. Diseases of Aquatic Organisms 102:169–180.
Rollins-Smith, L.A., and E.H. Le Sage. 2021. Batrachochytrium fungi: stealth invaders in amphibian skin. Current Opinion in Microbiology 61:124–132.
Skelly, D.K., E.E. Werner, and S.A. Cortwright. 1999. Long-term distributional dynamics of a Michigan amphibian assemblage. Ecology 80:2326–2337.
Snodgrass, J.W., R.E. Casey, J.A. Simon, and K. Gangapura. 2008. Ecotoxicology of amphibians and reptiles in urban environments: an overview of potential exposure routes and bioaccumulation. Pages 177–196 in J. C. Mitchell, R. E. Jung Brown, and B. Bartholomew, editors. Urban Herpetology. Society for the Study of Amphibians and Reptiles, Salt Lake City, Utah, USA.
Staudinger, M.D., A.V. Karmalkar, K. Terwilliger, K. Burgio, A. Lubeck, H. Higgins, T. Rice, T.L. Morelli, A. D'Amato. 2024. A regional synthesis of climate data to inform the 2025 State Wildlife Action Plans in the Northeast U.S. DOI Northeast Climate Adaptation Science Center Cooperator Report. 406 p. <https://doi.org/10.21429/t352-9q86>
Stockwell, S.S., and M.L. Hunter Jr. 1989. Relative abundance of herpetofauna among eight types of Maine peatland vegetation. Journal of Herpetology 23:409–414.
Sutherland, R.W., P.R. Dunning, and W.M. Baker. 2010. Amphibian encounter rates on roads with different amounts of traffic and urbanization. Conservation Biology 24:1626–1635.
Tennessen, J.B., S.E. Parks, and T. Langkilde. 2014. Traffic noise causes physiological stress and impairs breeding migration behavior in frogs. Conservation Physiology 2(1). doi:10.1093/conphys/cou032. <http://conphys.oxfordjournals.org/content/2/1/cou032.full>
Voordouw, M.J., D. Adama, B. Houston, P. Govindarajulu, and J. Robinson. 2010. Prevalence of the pathogenic chytrid fungus, Batrachochytrium dendrobatidis, in an endangered population of northern leopard frog, Rana pipiens. BMC Ecology 10:6.
Walls, S.C., W.J. Barichivich, and M.E. Brown. 2013. Drought, deluge, and declines: the impact of precipitation extremes on amphibians in a changing climate. Biology 2013:399–418.
Wyman, R.L. 1988. Soil acidity and moisture and the distribution of amphibians in five forests of southcentral New York. Copeia 1988:594–599.
Contact
| Date published: | April 11, 2025 |
|---|