- Scientific name: Gyrinophilus porphyriticus
- Species of Greatest Conservation Need (MA State Wildlife Action Plan)
Description
Spring salamander, sometimes called “Purple salamander” or “Gyro,” is the largest of the lungless salamanders (Family Plethodontidae) in New England, with adults measuring 11-21 cm (4.3-8.3 in) in total length. Base coloration in adults ranges from salmon or pinkish-orange in younger individuals to dark orange and brownish-maroon in older individuals. In all adults, the body and tail are marked with thin, dark-colored streaks and mottling that appear to form a reticulate or net-like pattern, which darkens and becomes more prominent with age. There are 17-19 costal grooves. Perhaps the most diagnostic physical characteristic of spring salamander is a light-colored line extending from each eye to the tip of the snout along a ridge called the “canthus rostralis.” No other salamander species native to Massachusetts has that marking.
Larvae are pale (whitish-beige in hatchlings to lavender-gray in individuals of moderate age) and have external, plume-like gills that may appear reddish, grayish, or brownish. The body and tail have a faint reticulate pattern. Mature larvae nearing metamorphosis may appear yellowish-brown, olive-colored, or orangish-brown with a prominent reticulate pattern before completing metamorphosis and transitioning to the salmon color of young adults. The snout in larvae is relatively long and squared – almost shovel-like – compared with other stream salamander species of Massachusetts, and the small eyes are set forward. Hatchlings are 18-26 mm (0.7-1.0 in) in total length and eventually grow to the size and length of a small adult before metamorphosis.
A typical spring salamander larva of moderate age. Note the lavender-gray coloration, faint reticulate pattern, and shovel-like snout. In this individual, the external gills are the same color as the body and, therefore, barely visible from this angle.
Life cycle and behavior
In Massachusetts and other parts of the Northeast, spring salamanders are able to complete their life cycle within streams, springs, seeps, and the immediately bordering uplands. These salamanders are seldom observed more than several meters from their home stream or spring.
Winters and periods of low or depleted surface water are spent in subterranean retreats such as groundwater crevices below the streambed, or in similar spaces among stones and gravel in the streambank where temperature and moisture conditions are suitable. During other times of the year, larvae and adults may dwell within surface waters (beneath stones or on the bottom), or adults may sit beneath stones, logs, or other objects at the edge of the water or just beyond. On wet nights, adults may emerge to forage in the open, either along or just beyond their spring, seep, or streambed. Although spring salamander is known to be a heavy consumer of other salamanders in southern states, the diet of northern populations consists mainly of invertebrates. Adults consume both aquatic and terrestrial prey, with common items including leafhoppers, slugs, beetles, snails, mayflies, caddisflies, flies, earthworms, spiders, millipedes, and hymenopterans. Larvae feed most commonly on aquatic invertebrates (fly larvae, caddisfly larvae, stonefly larvae, hydrophilid beetle larvae) and northern two-lined salamander (Eurycea bislineata) larvae. As a gape-limited predator, spring salamanders will eat just about any organism small enough to swallow.
Mating has seldom if ever been observed in the wild. Evaluations of male anatomy, presence of sperm in females, and the timing of observations of eggs in streams suggest that the breeding season of spring salamander may be during the spring. However, many of those data are from southern states, and there is evidence from New York populations that mating may occur in the fall. Despite the dearth of mating observations, there is information about courtship behavior from wild-captured adults brought into the lab. Courtship begins with the male approaching the female and contacting her with his snout before proceeding to rub his head laterally over her body, head, and snout. If she is receptive, she does not retreat, and the male positions himself in front of her, with the end of his tail below her head. The two then engage in a “tail-straddle walk” where the female moves over the tail of the male and straddles it as the two move forward in tandem. The male eventually deposits a spermatophore (a tiny packet of sperm) on the substrate and flexes his tail at a 90-degree angle. After he moves forward, the female follows and pauses to pick up the spermatophore with her cloaca, drawing it into her body. Her eggs are later fertilized.
Timing of the spring salamander breeding season at northern latitudes is not well understood, as courtship is seldom (if ever) observed in the wild. This pair of adults found beneath a stone in Williamstown, Massachusetts, might suggest a possibility of autumn pairings.
The “nest” consists of a clutch of eggs attached in a continuous monolayer to the underside of a large stone in running water. Eggs of spring salamander are seldom encountered, and so most are assumed to be deposited in groundwater recesses. Egg deposition can occur as early as May, but summer is more typical. The mature ova are light yellow in color, 3.5-4.0 mm (0.14-0.16 in) in diameter, and surrounded by three gelatinous layers that result in a total egg diameter of approximately 9 mm (0.35 in). Clutch size varies by the size (and presumably age and body condition) of the female. In a small sample of dissected spring salamanders in New York and Pennsylvania, females carried 44-132 ova. Observed nests have included clutch sizes of 15-66 eggs. The female may guard the eggs until they hatch in late summer or early fall.
Hatchlings are entirely aquatic and believed to remain in the immediate vicinity of the nest for some time – possibly through the winter – before dispersing into other areas of the stream or spring. Larvae are secretive during the day and dwell beneath partially or wholly submerged stones or even in groundwater spaces below the streambed. At night, larvae may be encountered foraging on the streambed. The aquatic larval period can be variable but lasts at least several years; 4 years is believed to be most common. The larvae attain a total length of 10-14 cm (3.9-5.5 in) at metamorphosis, which typically occurs in late spring or summer.
Sexual maturity may be attained shortly after metamorphosis or delayed up to a year. Maximum life expectancy in the wild is not known, but there is one report of a spring salamander having lived 18.5 years in captivity.
Distribution and abundance
Spring salamander ranges from western Maine and southern Quebec south through the Appalachian Mountains and foothills to northcentral Alabama and extreme northeastern Mississippi; it is absent from the Atlantic Coastal Plain. Within Massachusetts, spring salamander occurs in the 5 westernmost counties: Berkshire, Franklin, Hampden, Hampshire, and Worcester. Most populations are concentrated in the northwestern quadrant of the state, with scattered population occurring as far south as Mount Washington and Granville and as far east as Sutton and Leominster. As of February 2025, populations from 48 towns had been documented and reported to MassWildlife’s Natural Heritage & Endangered Species Program (NHESP) since 2000.
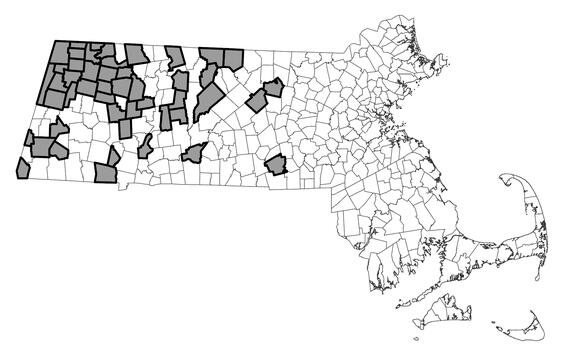
Distribution in Massachusetts.
2000-2024
Based on records in the Natural Heritage Database.
Population status
Formerly listed as special concern pursuant to the Massachusetts Endangered Species Act (M.G.L. c. 131A) and implementing regulations (321 CMR 10.00), spring salamander was “delisted” (i.e., removed) from the Massachusetts List of Endangered, Threatened, and Special Concern Species in 2006. The delisting, which followed an assessment in 2005, was based largely on a determination that a sufficient number of local populations had been identified on protected lands to conclude that the species was not at risk of becoming threatened with extinction in Massachusetts. The NHESP subsequently conducted a follow-up population assessment during 2019–2020. Although the geographic distribution of the species generally remained unchanged from 2005, surveys failed to detect spring salamanders at some sites of previously documented occurrence, suggesting a possibility of local extirpations. Furthermore, habitat degradation was observed at a number of sites. Therefore, spring salamander remains a species of conservation interest in Massachusetts. It is legally protected from being disturbed, harassed, or taken pursuant to 321 CMR 3.05.
Habitat
Massachusetts populations of spring salamander most commonly occur in clear, cool, well-oxygenated streams of forested, hilly terrain. Springs are used, as are seepages near streams. Larvae are fully aquatic, and the lungless adults are both aquatic and terrestrial. One of the most important elements of spring salamander stream habitat is an abundance of large, flat stones mixed with beds of gravel and cobble where larvae and adults alike can dwell in interstitial water. Accordingly, there is little accumulation of fine, silty, organic matter. Waters are alkaline to slightly acidic. Deciduous broadleaf forest is believed to provide the best surrounding terrestrial habitat, though some spring salamander streams are bordered by stands of Eastern Hemlock (Tsuga canadensis).
Healthy habitats are vital for supporting native wildlife and plants. Explore habitats and learn about conservation and restoration in Massachusetts.
A typical spring salamander stream in Massachusetts. Note its rockiness and the lack of organic silt or sand deposits.
Headwater (i.e., first-order) streams support the highest densities of spring salamander, especially when one of its main predators, brook trout (Salvelinus fontinalis), is absent. Spring salamanders do co-occur with brook trout in higher-order streams where – in addition to their predator-prey relationship – they compete for food and for space beneath rocks. Crayfish (e.g., Cambarus bartonii) and green frogs (Lithobates clamitans) are additional competitors for space beneath rocks in spring salamander streams.
Upper reaches of headwater streams – where Brook Trout are usually absent – tend to support the highest densities of spring salamander in the Northeast.
Threats
Primary threats to spring salamander in Massachusetts are habitat degradation, climate change, and anthropogenic disturbance. These threats may act alone or in combination to cause direct, indirect, and/or cumulative impacts to a given population.
Habitat degradation
The main forms of habitat degradation in Massachusetts are sedimentation and warming of streams. Sedimentation occurs when sand from roadways or soils from woodland trails wash into streams and fill spaces beneath and around stones in the streambed, accumulating to the point that most stones become embedded or buried. Interstitial spaces between stones and the streambed are critical microhabitats for spring salamanders to avoid predators and stay hydrated while resting and feeding; populations suffer when these spaces are lost. The most common causes of stream sedimentation in spring salamander habitat appear to be poor road management practices (e.g., winter sanding above steep embankments and at stream crossings, diversion of runoff toward streams); operation of logging equipment over unstable soils (especially in headwater areas and at stream crossings); and off-road vehicle use near or through streams.
Spring salamanders also depend on cool, well oxygenated water, and so an increase in water temperature is another threat to habitat quality. The biggest threats to water temperature in spring salamander streams are climate change, insufficient canopy retention during logging operations, and multiple crossings of a stream by roads and utility rights-of-way.
This tributary of a spring salamander stream in western Massachusetts was destroyed when roadway managers graded a water diversion feature to direct runoff directly into the stream.
Climate change
A 2024 synthesis of climate data, climate modeling, and climate-related research indicates that temperature, total annual precipitation, and frequency of heavy precipitation events are trending upward in the northeastern United States and are expected to continue to do so into the future. Those trends are expected to negatively impact spring salamander populations. Warming of surface waters would reduce dissolved oxygen concentrations, which could potentially increase salamander stress, reduce foraging efficiency, and/or force salamanders to spend more time beneath the streambed and, therefore, less time feeding. Increased precipitation is likely to increase average water flows, which may consequently reduce foraging opportunity within the streambed. One study of a New Hampshire population has suggested that an observed decline in its adult population is linked to climate change, particularly with respect to an increasing frequency of unusually strong precipitation events. The study attributes the decline to reduced recruitment and suggests a possibility that mature larvae are incurring high rates of mortality when rapid and violent flooding coincides with the final stages of salamander metamorphosis, when microhabitat stability is especially important.
Anthropogenic disturbance
At a much smaller scale, spring salamanders are vulnerable to anthropogenic disturbance. Recreationists that search for salamanders outside of state-coordinated surveys disturb and displace salamanders, put salamanders at risk of physical injury (e.g., when moving large stones), and disturb important microhabitat – all without any mitigating benefit to the salamander population. This type of disturbance may be especially problematic near popular hiking trails on public land.
Conservation
Some prospective conservation measures for spring salamander in Massachusetts may be grouped into three general categories: inventory and monitoring, management, and research. The NHESP is the state authority for coordinating and implementing such measures, often in collaboration with other agencies, academic institutions, land trusts, municipal conservation departments, private-sector herpetologists, and others. The NHESP also facilitates participation by the general public.
Inventory and monitoring
Inventory surveys are essential to discovering undocumented populations of spring salamander on the Massachusetts landscape and to evaluating the relative robustness of each. Knowledge about population abundance and distribution is prerequisite to understanding the conservation status of the species and to making informed decisions about how and where to invest scarce conservation resources. The NHESP, its collaborators, and the public have made strong gains in documenting populations of spring salamander and understanding the state distribution of the species over the past several decades, but undoubtedly there are additional populations to be found and evaluated.
Periodic monitoring surveys are necessary to stay informed about population statuses over time. These surveys provide insight about the effectiveness of certain management strategies, and they serve to detect site-specific threats or signs of population decline or extirpation.
Management
At a local scale, sites of known occurrence of spring salamander should be managed to develop or maintain mature forest conditions to help maintain soil stability and cool water temperatures. Percent canopy cover of native tree species should be maximized within the first 30-60 m (100-200 ft) beyond a known or likely spring salamander stream, spring, or seep. Maintenance of closed canopy, mature forest conditions beyond 60 m (200 ft) may be beneficial where slopes are especially steep or in the area surrounding a network of headwater seepages and streams. Forest cover type should be managed to maintain and/or enhance dominance by deciduous broadleaf species, though eastern hemlock is naturally common – or even dominant – at some spring salamander sites and should not be targeted for removal. Wintertime sanding of public roads should not be implemented at or near stream crossings or along stretches of road that closely parallel spring salamander streams (e.g., within 30 m [100 ft] of low-gradient reaches or within 60 m [200 ft] of high-gradient reaches). Dirt roads should not be graded or otherwise engineered to direct surface runoff into or toward streams without the use (and regular maintenance) of effective buffers to slow the flow of water and remove suspended sediments. Within the forest, logging equipment and recreational trails should avoid crossing streams to the extent possible and, when unavoidable, engineered to ensure soil stability. Recreational trails should be set back at least 30 m (100 ft) from streambeds, with stream-viewing or access opportunities managed via strategically placed and well-defined spur trails. Existing trails that traverse stretches of streambank should be relocated and allowed to revegetate. Any crossing of a spring salamander stream by a public road or private woods road should be equipped with a culvert whose diameter (or width) encompasses the full width of the streambed, and the bottom of the culvert should be lined inside with a layer or two of loose, variably sized stones.
At the landscape scale, land area of mature upland forest around spring salamander streams should be maximized. Land acquisition and protection efforts should prioritize sites with networks of headwater streams over sites having just a single stream flowing directly into a high-order stream. Land protection should also prioritize landscapes with low road densities, targeting stream networks that are relatively distant (e.g., greater than 100 m [330 ft]) from the nearest road or development. Lastly, areas with relatively cool microclimate should be prioritized over warmer sites that are less likely to remain suitable with climate change.
Research
Research is necessary to help fill or improve upon knowledge gaps that might otherwise limit the effectiveness of current practices in conservation planning and management. We will never know everything about spring salamander, but some general topics of conservation research interest include:
- Trends in stream temperature, dissolved oxygen, and hydrological regime in association with climate change and spring salamander occupancy;
- Development of non-invasive methods of spring salamander population monitoring;
- Development of monitoring techniques that can account for inaccessible salamanders (e.g., those beneath immovable stones or below the streambed); and
- Behavioral ecology of spring salamanders and their common competitors for streambed refugia.
Evaluations of spring salamander abundance are very difficult when the predominant cover objects in a stream consist of immovable stones, like at this site in Russell, Massachusetts.
Community participation
The general public is encouraged to participate in the conservation of spring salamander primarily by reporting incidental observations of the species to the NHESP, as status assessment and land-protection efforts for spring salamander are dependent on knowing where local populations occur and maintaining current observation records. However, due to the reclusive nature of the species and the sensitivity of its microhabitat, members of Massachusetts community are advised not to actively search for the species outside of a survey coordinated with the NHESP (in fact, spring salamander is a protected species and not to be taken, disturbed, or harassed without a permit from MassWildlife). Lack of coordination among surveyors may accidentally result in duplicative survey efforts and unnecessary disturbance to salamanders and their habitat.
Landowners in spring salamander habitat are encouraged to manage their properties for mature, closed-canopy forest and protection of stream water quality as described in the “Management” subsection above. They may also consider placing a conservation restriction on their properties to help ensure long-term protection of salamander habitat.
Lastly, spring salamander has been known to dwell within springhouses on occasion. Landowners with a springhouse in Worcester County westward – especially if it occurs near a forested hillside containing a stream – are encouraged to preserve the structure to the extent possible.
References
Barret, K., B.S. Helms, C. Guyer, and J.E. Schoonover. 2010. Linking process to pattern: causes of stream-breeding amphibian decline in urbanized watersheds. Biological Conservation 143:1998–2005.
Beachy, C.K. 1997. Courtship behavior in the plethodontid salamander Gyrinophilus porphyriticus. Herpetologica 53:289–296.
Bishop, S.C. 1941. The salamanders of New York. New York State Museum Bulletin 324:1–365.
Bruce, R.C. 1972. Variation in the life cycle of the salamander Gyrinophilus porphyriticus. Herpetologica 28:230–245.
Bruce, R.C. 1980. A model of the larval period of the spring salamander, Gyrinophilus porphyriticus, based on size-frequency distributions. Herpetologica 36:78–86.
Burton, T.M. 1976. An analysis of the feeding ecology of the salamanders (Amphibia, Urodela) of the Hubbard Brook Experimental Forest, New Hampshire. Journal of Herpetology 10:187–204.
Deitchler, E.A., J.M. Davenport, and W.H. Lowe. 2015. Homing behavior of the northern spring salamander, Gyrinophilus porphyriticus, in a northeastern United States headwater stream. Herpetological Conservation and Biology 10:235–241.
Fields, W.R, E.H.C. Grant, and W.H. Lowe. 2017. Detecting spatial ontogenetic niche shifts in complex dendritic ecological networks. Ecosphere 8(2):e01662. 10.1002/ecs2.1662.
Grant, E.H.C., L.E. Green, and W.H. Lowe. 2009. Salamander occupancy in headwater stream networks. Freshwater Biology 54:1370–1378.
Greene, B.T., W.H. Lowe, and G.E. Likens. 2008. Forest succession and prey availability influence the strength and scale of terrestrial-aquatic linkages in a headwater salamander system. Freshwater Biology 53:2234–2243.
Klemens, M.W., H.J. Gruner, D.P. Quinn, and E.R. Davison. 2021. Conservation of amphibians and reptiles in Connecticut. Department of Energy and Environmental Protection, Hartford, Connecticut, USA.
Lowe, W.H. 2003. Linking dispersal to local population dynamics: a case study using a headwater salamander system. Ecology 84:2145–2154.
Lowe, W.H. 2009. What drives long-distance dispersal? A test of theoretical predictions. Ecology 90:1456–1462.
Lowe, W.H. 2010. Explaining long-distance dispersal: effects of dispersal distance on survival and growth in a stream salamander. Ecology 91:3008–3015.
Lowe, W.H. 2012. Climate change is linked to long-term decline in a stream salamander. Biological Conservation 145:48–53.
Lowe, W.H. and D.T. Bolger. 2002. Local and landscape-scale predictors of salamander abundance in New Hampshire headwater streams. Conservation Biology 16:183–193.
Lowe, W.H., G.E. Likens, M.A. McPeek, and D.C. Buso. 2006. Linking direct and indirect data on dispersal: isolation by slope in a headwater stream salamander. Ecology 87:334–339.
Lowe, W.H., K.H. Nislow, and D.T. Bolger. 2004. Stage-specific and interactive effects of sedimentation and trout on a headwater stream salamander. Ecological Applications 14:164–172.
Petranka, J.W. 1998. Salamanders of the United States and Canada. Smithsonian Institution Press, Washington, D.C., USA.
Raithel, C.J. 2019. Amphibians of Rhode Island. Rhode Island Division of Fish and Wildlife, West Kingston, USA.
Resetarits, W.J., Jr. 1995. Competitive asymmetry and coexistence in size-structured populations of brook trout and spring salamanders. Oikos 73:188–198.
Snider, A.T. and J.K. Bowler. 1992. Longevity of reptiles and amphibians in North American collections. Second edition. Society for the Study of Amphibians and Reptiles, Herpetological Circular No. 21.
Staudinger, M.D., A.V. Karmalkar, K. Terwilliger, K. Burgio, A. Lubeck, H. Higgins, T. Rice, T.L. Morelli, A. D'Amato. 2024. A regional synthesis of climate data to inform the 2025 State Wildlife Action Plans in the Northeast U.S. DOI Northeast Climate Adaptation Science Center Cooperator Report. 406 p. <https://doi.org/10.21429/t352-9q86>
Ward, R.L., J.T. Anderson, and J.T. Petty. 2008. Effects of road crossings on stream and streamside salamanders. Journal of Wildlife Management 72:760–761.
Contact
| Date published: | April 11, 2025 |
|---|