- Scientific name: Enallagma daeckii
- Species of Greatest Conservation Need (MA State Wildlife Action Plan)
- Threatened (MA Endangered Species Act)
Description
Male (top) and female (bottom) attenuated bluets
The attenuated bluet is a small, semi-aquatic insect of the order Odonata, suborder Zygoptera (the damselflies), and family Coenagrionidae (pond damsels). Like most damselflies, attenuated bluets have large eyes on the sides of the head, short antennae, and four heavily veined wings that are held folded together over the back. The attenuated bluet is characterized by having an exceptionally long, slender abdomen. On average, it is the longest pond damsel in the United States. The male’s thorax (winged and legged section behind the head) is mostly pale blue with thin black stripes on the “shoulders” and top. The abdomen, which is composed of ten segments, is mostly dark brown/black with some blue on the sides of the base of the abdomen and an entirely blue tip (half of segment 7 and all of segments 8-10). Females have thicker abdomens than the males, and are generally brown where the males are blue, though older females may become quite bluish. Attenuated bluets range from 38-46 mm (1.5-1.8 in) in length.
The bluets (genus Enallagma) comprise a large group of damselflies, with more than 20 species in Massachusetts. Identification of the various species can be very difficult and often requires close examination of the terminal appendages on the males (Nikula et al. 2007) or the mesostigmal plates (located behind the head) on the females (Westfall & May 1996). The attenuated bluet is most similar in appearance to the more common and widespread slender bluet (E. traviatum). The two species are most safely distinguished by the shape of the terminal appendages on the male and the mesostigmal plates of the females. Attenuated bluets have much longer abdomens, giving them a lankier appearance than slender bluets. However, this feature may require direct comparison between species and there is some variation in size so it is not entirely reliable for identification.
Life cycle and behavior
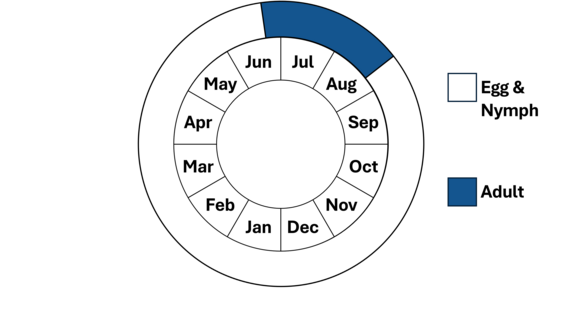
Note adult life stage is synonymous with fight period.
Although little has been published specifically on the life history of the attenuated bluet, it is likely similar to other, better-studied species in the genus. All odonates have three life stages: egg, aquatic nymph, and flying adult. The nymphs are slender with three leaf-like appendages extending from the end of the body which serve as breathing gills. They have a large, hinged lower jaw which they can extend forward with lightning speed. This feature is used to catch prey, the nymph typically lying in wait until potential prey passes within striking range. They feed on a wide variety of aquatic life, including insects and worms. They spend most of their time clinging to submerged vegetation or other objects, moving infrequently. They transport themselves primarily by walking but are also capable of swimming with a sinuous, snake-like motion.
Attenuated bluets have a one-year life cycle. The eggs are laid in the summer and probably hatch in the fall. The nymphs develop over the winter and spring, undergoing several molts. In early to mid-summer the nymphs crawl up on emergent vegetation and begin their transformation into adults. This process, known as emergence, typically takes a couple of hours, after which the newly developed adults (tenerals) fly weakly off to upland areas where they spend a week or two feeding and maturing. The young adults are very susceptible to predators, particularly birds, ants, and spiders; mortality is high during this stage of the life cycle. The adults feed on a wide variety of smaller insects that they typically catch in flight.
When mature, the males return to the wetlands where they spend most of their time searching for females. When a male locates a female, he attempts to grasp her behind the head with the terminal appendages at the end of his abdomen. If the female is receptive, she allows the male to grasp her, then curls the end of her abdomen up to the base of the male’s abdomen where his secondary sexual organs (hamules) are located. This coupling results in the heart-shaped tandem formation characteristic of all odonates. This coupling lasts for a few minutes to an hour or more. The pair generally remains stationary during this mating but, amazingly, can fly, albeit weakly, while coupled.
Once mating is complete, the female begins laying eggs (oviposits) in emergent grasses and rushes, using the ovipositor located on the underside of her abdomen to slice into the vegetation and deposit eggs. Although the female occasionally oviposits alone, in most cases the male remains attached to the back of the female's head. This form of mate-guarding is thought to prevent other males from mating with the female before she completes egg-laying. The adult’s activities are almost exclusively limited to feeding and reproduction, and their life is short, probably averaging only three to four weeks. The flight season of attenuated bluet ranges from mid-June to mid-August with most observations in July.
Distribution and abundance
The attenuated bluet ranges from Massachusetts south to Florida and west to Indiana, Oklahoma and Texas. The attenuated bluet reaches the edge of its range in New England, and has been recorded Connecticut, Rhode Island, and Massachusetts. In Massachusetts, the species is restricted to the coastal plain and occurs in the Cape Cod, Tanton, Neponset, and Blackstone watersheds with most observations in the former two. Recent surveys have documented new attenuated bluet occurrences on the outer half of Cape Cod. Despite seemingly identical habitat, the species was not detected in many ponds in the inner half of Cape Cod and in Plymouth County (Nikula 2019); however, a few newer occurrences have been documented more inland. Patterns of the species distribution likely reflect both survey effort and colonization patterns from more southern populations (e.g. Rhode Island) and may represent an ongoing range expansion.
The attenuated bluet is listed as a Threatened species in Massachusetts and is protected under the Massachusetts Endangered Species Act (MG.L. c.131A) and its implementing regulations (321 CMR 10.00). As with all species listed in Massachusetts, individuals of the species are protected from take (picking, collecting, killing, etc.) and sale under the Massachusetts Endangered Species Act.
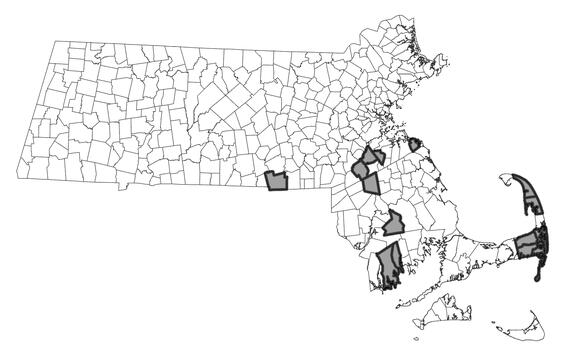
Distribution in Massachusetts.
1999-2024
Based on records in the Natural Heritage Database.
Habitat
Attenuated bluets inhabit a variety of lentic systems that are typically with abundant emergent, floating and submerged aquatic vegetation. Wetland habitats include coastal plain ponds, impoundments, stream backwaters, and swamps. The nymphs are aquatic and live among aquatic and emergent vegetation and debris. The adults inhabit nearby forested uplands and emergent vegetation along the shore where they are often observed.
Healthy habitats are vital for supporting native wildlife and plants. Explore habitats and learn about conservation and restoration in Massachusetts.
Coastal plain pond nearshore habitat suitable for attenuated bluet. Credit: Jason Carmignani.
Threats
The major threats to attenuated bluet are shoreline and wetland degradation and loss. Shoreline development may eliminate nearshore/littoral zones and riparian vegetation and harden shorelines through construction of buildings, roads, and other human constructions. Further, shoreline development facilitates increased nutrient and contaminant inputs (e.g., road salts, septic, fertilizer), sedimentation, surface and groundwater withdrawals or water level alteration (e.g., winter drawdown), pesticide use, introduction and spread of invasive species (aquatic vegetation and animals), and recreational activity (e.g., off-road vehicles, boat wakes). These activities lead to wetland/pond degradation that accelerates eutrophication, degrades water quality, and alters or eliminates aquatic vegetation composition required for attenuated bluet including sedges, rushes, and grasses. Invasive species including Phragmites can replace native emergent vegetation creating unsuitable habitat conditions for pine barrens bluet. In addition, climate change may create unfavorable conditions, including prolonged drought and high-water events, that in combination with ongoing habitat degradation (water withdrawals, nutrient inputs) can increase cyanobacteria blooms, reduce habitat, and alter aquatic vegetation composition unsuitable for the species. High-impact recreational use such as off-road vehicles driving through pond shores, which may destroy breeding and nymphal habitat, and motorboats, whose wakes swamp delicate emerging adults, are also threats. Since attenuated bluets, spend a period of several days or more away from the pond maturing, it is important to maintain natural upland habitats adjoining the breeding sites for roosting and hunting. Without protected uplands the delicate newly emerged adults are more susceptible to predation and mortality from inclement weather (Hunt et al. 2020).
Conservation
Survey and monitoring
Standardized surveys should target known sites and new wetlands to determine attenuated bluet occupancy and population status. Surveys for adults are likely to be more effective for detection compared to nymph or exuvia as this life stage is extremely difficult to find and identify. Adult surveys should target nearshore and riparian habitats during their flight period during standardized weather and time windows to maximize species detection. Multiple site visits (e.g., ≥3) are likely required to detect this species because of its typical low abundances and ephemerality. Known sites with breeding evidence should be monitored every 5 years or as needed (i.e., in response to extreme conditions like drought) to document changes in occupancy and habitat conditions. Effort should also be devoted to surveys at potential suitable sites to document potential dispersal and range expansion and update the species distribution and status in the state.
Management
Protection and restoration of shoreline/littoral zone, riparian, and upland habitat is critical for attenuated bluet persistence in Massachusetts. Actions that can improve or prevent habitat degradation include: reduction of nutrient, agricultural and road runoff; minimization of water level alteration that impacts native aquatic vegetation; minimization of groundwater withdrawals particularly during drought periods; prevention and management of nonnative aquatic vegetation; development of best practices for herbicide use; limitation and prevention of off-road vehicles access on shoreline habitat; and connection between ponds and other pond complexes.
Research needs
Research effort is needed to estimate detection and occupancy rates and how other environmental variables (e.g., sample timing, weather) affect these rates. Identification of source and sink wetland sites and general population dynamics within and across coastal plain ponds complexes is needed in Massachusetts to prioritize site conservation. Other research efforts include estimation of physiological tolerances to insecticides and herbicides; impacts of non-native fish and aquatic vegetation on populations; and projections of species distribution under climate change scenarios and climate vulnerability analysis.
References
Brown, V.A. 2020. Dragonflies and Damselflies of Rhode Island. Rhode Island Division of Fish and Wildlife, Department of Environmental Management, West Kingston RI.
Hunt, P. 2020. Conservation planning for endemic damselflies of the northeast: A report to the Sarah K. deCoizart Article TENTH Perpetual Charitable Trust. Concord, NH. 18 p.
Hunt, P., V. Brown, R. Butler, P. deMaynadier, L. Harper, L. Saucier, R. Somes, E. White. 2020. A conservation plan for the endemic damselflies of the northeast. 20 p.
Lam, E. 2004. Damselflies of the northeast. Biodiversity Books, Forest Hills, New York, 96 p.
Nikula, B. 2019. A survey for five species of Enallagma (Bluet) damselflies in southeastern Massachusetts. Report to Natural Heritage and Endangered Species Program, Massachusetts Division of Fisheries and Wildlife, Westborough, MA.
Nikula, B., J.L. Ryan, and M.R. Burne. 2007. A Field Guide to the Dragonflies and Damselflies of Massachusetts. Massachusetts Natural Heritage and Endangered Species Program.
Walker, E.M. 1953. The Odonata of Canada and Alaska, Vol. I. University of Toronto Press.
Westfall, M.J., Jr., and M.L. May. 1996. Damselflies of North America. Scientific Publishers.
Contact
| Date published: | April 3, 2025 |
|---|