- Scientific name: Phanogomphus descriptus
- Species of Greatest Conservation Need (MA State Wildlife Action Plan)
- Endangered (MA Endangered Species Act)
Description
Male harpoon clubtail
The harpoon clubtail (Phanogomphus descriptus) is a large, slender insect belonging to the order Odonata, suborder Anisoptera (the dragonflies), and family Gomphidae (the clubtails). The clubtails are a large, diverse group of dragonflies named for the lateral swelling at the tip of the abdomen that produces a club-like appearance which varies greatly among species. Clubtails are further distinguished from other dragonflies by their widely separated eyes, wing venation characteristics, and behavior. The harpoon clubtail belongs to the genus Phanogomphus (American clubtails) that are characterized by their dull coloring of grays, greens, browns and blacks, and by their relatively small clubs. Harpoon clubtails have a plain gray-green face and eyes that range in color from pale to deep aqua blue. The thorax (section behind the head) is marked laterally with three wide grayish-green stripes and two reduced dark stripes. Dorsally, the thorax is marked with two gray-green stripes with posterior hooks. The abdomen (section behind the thorax) is almost completely with thin, grayish-green, dorsal stripes on segments one through seven and lateral small lateral blotches on segments eight and nine. Recently emerged individuals are more brightly colored than mature individuals and can initially cause identification problems. Females are similar in coloration but have more extensive yellow markings on the dorsal and lateral sides of the abdomen and are stouter overall except for the club.
Adult harpoon clubtails range from about 46-51 mm (1.8 -2 in) in length. The fully developed nymphs range from 29-31mm (1.1-1.2 in).
Although one can fairly quickly recognize a clubtail as belonging to the genus Phanogomphus by a combination of factors, including its coloration and small club, members of this genus can be very difficult to identify to the species level. Examination of the terminal abdominal appendages of the male and the vulvar lamina of the female are the most reliable method for identification. Species with the most similar appearance include beaverpond clubtail (P. borealis) and rapids clubtail (P. quadricolor). Male beaverpond clubtail lacks posterior hooks on dorsal stripes and rapids with lateral abdominal markings, and both with differently shaped hamules than harpoon clubtail. Female harpoon clubtails have more extensive lateral abdominal markings than rapids clubtail and have femur hindleg is pale compared to the black legs of beaverpond clubtail females. The nymphs and exuviae can be distinguished by characteristics of the labium as per the keys by Walker (1958), and Soltesz (1996), and Tennessen (2019).
Life cycle and behavior
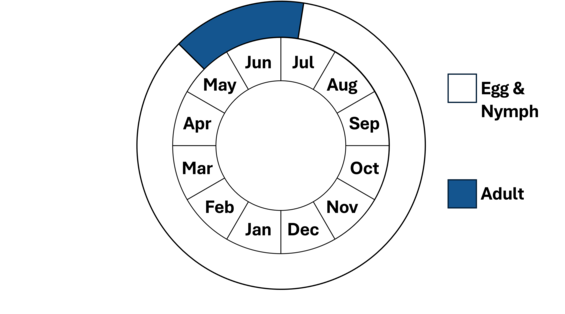
Note, nymphs are present year-round.
Harpoon clubtails are elusive and little is known about their life history. The egg and nymph life stages are fully aquatic. The nymphs are predators, feeding on a wide variety of aquatic insects, small fish, and tadpoles. Nymphs spend much of their time burrowing in the bottom sediment in depositional habitats that provide them protection from predators and camouflage to catch prey. Nymphs undergo several molts (instars) for at least 2 years until they are ready to emerge as winged adults. When ready to emerge, typically in late May, the nymphs crawl out onto exposed rocks, emergent vegetation, partially submerged logs, or the steeper sections of riverbanks, where they undergo transformation to adults (a process known as eclosion). Emergence generally takes place very early in the morning, presumably to reduce exposure to predation. The cast nymphal exoskeletons, known as exuviae, are identifiable to species and can be a reliable, useful means to determine the presence of a species.
As soon as the freshly emerged adults are dry and the wings have hardened sufficiently, they fly off to seek refuge in the vegetation of adjacent uplands. Here, they spend several days or more feeding and maturing, before returning to their breeding habitats. During this time of wandering and maturation, harpoon clubtails can be found in fields and forest clearings, sometimes far away from the breeding site, perched horizontally on sunlit vegetation or the ground. From such perches, harpoon clubtails make periodic feeding forays during which they consume small aerial insects such as flies and mosquitoes. When the maturation process is complete, the adults return to the stream to breed. In Massachusetts, the harpoon clubtail breeds through the month of June. Although the nymphs are found in the quiet pools below areas of rapids, the adults prefer the swifter sections of the rivers. Upon returning to the stream, males can be found perching on rocks in these areas or on shoreline vegetation. From exposed perches they make patrols out over the water, often returning to the same or a nearby perch. During these patrols, the males are primarily searching for mates and driving off any potential competitors. Females spend little time around the breeding habitat, except during the brief time when they are ready to mate and lay eggs. When mating is completed, the female returns to the water to deposit her eggs. Female harpoon clubtails oviposit alone by tapping the tip of their abdomen to the surface of the water. This is usually done in the faster sections of the stream where there are rapids. Females fly rapidly back and forth over the water touching the surface of the water with the tip of her abdomen every few feet to release eggs. Upon being laid, it is likely that the eggs are carried downstream from the rapids and deposited in the pools where they hatch and the nymphs develop.
Harpoon clubtails have a rather short flight season with emergence beginning in late May and early June and the adults flying into July.
Distribution and abundance
The harpoon clubtail is found from Nova Scotia west to Ontario, south through the Appalachian region to North Carolina and Kentucky. In New England, the harpoon clubtail is found in Maine, New Hampshire and Vermont, south through western Massachusetts, and into extreme northwestern Connecticut. In Massachusetts, the species occurs in the Housatonic, Farmington, Westfield, Deerfield, and Swift River watersheds. As with many species of clubtails, population densities appear to be low. This may reflect the elusiveness of the adults or low detection rates, and/or actual low population sizes with typically low exuviae collected and adults observed. Recent exuviae surveys have expanded its distribution within extant river systems.
The harpoon clubtail is listed as an Endangered species in Massachusetts. As with all species listed in Massachusetts, individuals of the species are protected from take (picking, collecting, killing, etc.) and sale under the Massachusetts Endangered Species Act.
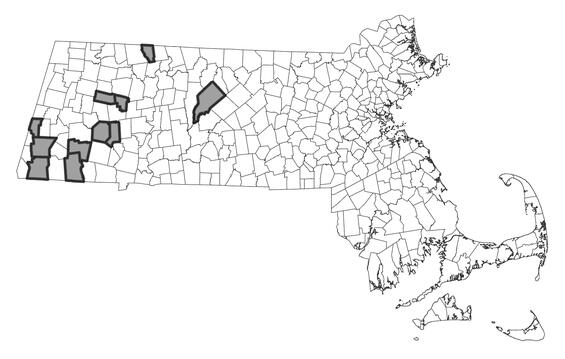
Distribution in Massachusetts.
1999-2024
Based on records in the Natural Heritage Database.
Habitat
The harpoon clubtail inhabits small to medium-sized streams that are typically clear and cold to cool in temperature. Substrates range from sand to boulders with intermittent riffles and rapids along with depositional mesohabitats. Exuviae have been typically found on banks adjacent to depositional mesohabitats including pools and slow runs. The adults inhabit riparian areas, forested uplands, fields, and when mature are often flying over swifter currents around riffles.
Healthy habitats are vital for supporting native wildlife and plants. Explore habitats and learn about conservation and restoration in Massachusetts.
Medium-sized stream with an extensive run habitat with mixed substrate of sand, gravel and cobble.
Threats
Degradation of water quality, alteration of streamflows, and upland habitat loss are primary threats to harpoon clubtail. Potential threats to water quality include pollution and sewage overflow, salt and other road contaminant run-off, and siltation from construction or erosion. The disruption of natural flow regimes by flow regulation projects, damming, and stream channelization may have a negative impact on populations. Warming stream temperatures from climate change is projected to reduce harpoon clubtail habitat in Massachusetts (Collins and McIntyre 2017). Additional threats illegal or accidental industrial discharge and hardening of channel banks and siltation that creates unstable stream habitat.
Conservation
Survey and monitoring
Since adults can be difficult to detect because of their elusive behavior, exuvia not only provides increased detection but also site-specific locations of aquatic nymph and breeding habitat.Surveys may target adults in combination with exuviae but never alone. Robust stream populations should be routinely monitored every five years or to the extent practical to document changes in occupancy and habitat conditions. Effort should also target new streams and stream segments identified as potentially suitable habitat. It’s possible the species occurs in more streams than currently known especially east of the Connecticut River.
Management
Upland and stream habitat protection is critical for the conservation of harpoon clubtail. Protection of forested upland borders of these river systems are critical in maintaining suitable water quality and are critical for feeding, resting, and maturation. Development of these areas should be discouraged, and the preservation of remaining undeveloped uplands should be a priority. Alternatives to commonly applied road salts should tested to minimize freshwater salinization. Hardened and channelized stream segments should be restored to promote natural sediment dynamics.
Research needs
Continued effort is needed to define habitat requirements, distribution, relative abundance, and potential breeding sites to update the species status in Massachusetts. Estimation of detection and occupancy rates and how other environmental variables (e.g., sample timing, weather) affect these rates for exuvia sampling is needed. Other research efforts include projections of species distribution under climate change scenarios and climate vulnerability analysis specifically in Massachusetts.
References
Collins S.D., and N.E. McIntyre. “Extreme loss of diversity of riverine dragonflies in the northeastern U.S. is predicted in the face of climate change.” Bulletin of American Odonatology 12,1 (2017):7-19.
Dunkle, Sidney W. Dragonflies Through Binoculars. Oxford University Press, 2000.
Lam, E. Dragonflies of North America. Princeton NJ: Princeton University Press, 2024.
Needham, J.G., M.J. Westfall, Jr., and M.L. May. Dragonflies of North America. Scientific Publishers, 2000.
Nikula, B., J.L. Ryan, and M.R. Burne. A Field Guide to the Dragonflies and Damselflies of Massachusetts. 2nd ed. Massachusetts Natural Heritage and Endangered Species Program, 2007.
Paulson, D. Dragonflies and Damselflies of the East. Princeton NJ: Princeton University Press, 2011.
Soltesz, K. Identification Keys to Northeastern Anisoptera Larvae. Center for Conservation and Biodiversity, University of Connecticut, 1996.
Tennessen, K. Dragonfly Nymphs of North America: An identification guide. Springer, 2019.
Contact
| Date published: | April 9, 2025 |
|---|