Introduction
Hepatitis C is a liver infection and inflammation caused by the hepatitis C virus (HCV). HCV is the most commonly reported bloodborne infection in the United States. Hepatitis C can be a serious disease resulting in long-term health problems, including liver damage, liver failure, cirrhosis, liver cancer, and even death. HCV is spread by direct contact with blood (or body fluids containing blood) of an infected person. This can happen through sharing equipment used to inject drugs or during birth to an HCV-infected mother. HCV may also be spread, though much less frequently, through unprotected sex with an HCV-infected individual, receipt of blood products or organ transplant (rare in the United States since 1992), receipt of tattoos or body-piercings with non-sterile equipment, sharing personal items contaminated with infected blood and needlestick injuries, or poor infection control in healthcare settings.
Many people who are newly infected with the hepatitis C virus (acute HCV) will have few or no symptoms, and therefore may be unaware of the infection. When symptoms of an acute HCV infection occur, they can include tiredness, loss of appetite, nausea, vomiting, stomachache, and muscle or joint pain. Urine may become darker in color, and jaundice (yellowing of the skin and whites of the eyes) may appear. Some who contract HCV will clear (get rid of) the virus without treatment, but over half of people infected with HCV will not clear the infection and go on to have chronic (long-term) infection. People with chronic HCV remain infectious and can pass it on to others. Most people with chronic HCV infection initially do not have any symptoms or have only nonspecific symptoms such as chronic fatigue and depression. However, many people with chronic HCV infection will gradually develop chronic liver disease, which can range from mild to severe and include cirrhosis (scarring of the liver) and liver cancer. Chronic HCV infection is the leading cause of liver cancer in North America, Europe, and Japan. Chronic liver disease caused by chronic HCV infection usually develops slowly, without any signs or symptoms, and progresses over several decades. Because of the long period during which people with chronic HCV infection can live without symptoms, a significant proportion (four in ten) with chronic HCV infection are undiagnosed and unaware of their infection. As a result, many people with chronic HCV infection are not accessing curative treatments to prevent disease progression and liver damage and are at risk of transmitting the virus to others. For this reason, the Centers for Disease Control and Prevention (CDC) recommends HCV screening at least once in a lifetime for all adults ages 18 years and older, and for all pregnant people during each pregnancy. Routine periodic HCV screening is recommended for people with ongoing risk factors, including injection drug use. In Massachusetts, not enough people are tested for HCV infection. Between 2016 and 2020, analysis of medical claims data shows that cumulatively, only 13.7% of residents had been tested at least once for HCV infection, and 2.3% had been tested two or more times. During this same period, approximately 3%-3.5% of Massachusetts residents were tested each year, with variations in uptake according to age, gender, and payer type.
Persons Who Use Drugs (PWUD), particularly those who inject drugs, face an elevated risk for infectious disease acquisition, including HIV, hepatitis A, hepatitis B, and bacterial infections including cellulitis, endocarditis, and bacteremia. In Massachusetts, 95% of confirmed cases of HCV reported between 2016 and 2020 with a known exposure mode, reported ever having injected drugs. Twenty percent of individuals diagnosed with HIV are co-infected with HCV; and among those with a history of injection drug use (IDU) the rate of co-infection is approximately 90%. While there are effective vaccines to protect against hepatitis A and B, there is currently no vaccine to prevent hepatitis C infection.
Blood tests show if a person has been exposed to the HCV virus (an antibody test) or if the person currently has hepatitis C infection (an RNA test). An RNA test following a positive antibody test is required to diagnose current HCV infection. CDC recommends that RNA testing be performed on reflex for all positive antibody tests. However, a 2022 DPH survey of clinical laboratories found that only 1/3 of clinical laboratories in Massachusetts conduct CDC-recommended reflex testing.
Direct acting antivirals (DAAs), highly effective oral medications, can cure HCV infection in almost all people in as little as eight weeks with minimal or no side effects. The American Association for the Study of Liver Disease (AASLD) and the Infectious Disease Society of America (IDSA) have identified several studies demonstrating the economic value of screening for HCV and providing HCV treatment to all people with infection, regardless of age or co-morbid conditions. Treatment as prevention of transmission of HCV, (with conclusive evidence being studied) is recognized as a potential elimination strategy. Yet data show that in Massachusetts, only about 4 of 10 people with chronic hepatitis C infection receive treatment within one year of diagnosis.
Massachusetts is in a strong position to eliminate Hepatitis C (HCV) infection as a public health threat to residents of the Commonwealth. This plan is ambitious. We outline a comprehensive set of core capacities and strategies that will increase awareness about HCV infection, expand access to prevention and treatment interventions, and improve health outcomes for individuals living with HCV. Our near- and longer- term objectives are as follows:
- Reduce new HCV infections by 20% by 2025 and 90% by 2030.
- Reduce new HCV infections among Hispanic/Lantinx individuals by 25% by 2025 and 90% by 2030
- Reduce new HCV infections among black/African American individuals by 25% by 2025 and 90% by 2030
- Increase HCV rates of cure to 58% by 2025 and 80% by 2030.
- Reduce rate of HCV-related deaths per 100,000 by 25% by 2025 and 65% by 2030.
The purpose of this document is to provide recommendations, objectives, and strategies regarding HCV prevention, diagnosis, and treatment to lead the Commonwealth toward eliminating HCV as a public health problem. The objectives and strategies will be revised and updated periodically as advances in prevention and treatment, as well as policy, transform HCV prevention and control in Massachusetts and the United States. The Massachusetts Department of Public Health (MDPH) Bureau of Infectious Disease and Laboratory Sciences (BIDLS) will internally assess progress on objectives and strategies described in this Plan. At the same time, BIDLS will acknowledge and continue to recognize complementary efforts and contributions from internal and external partners working to achieve HCV elimination.
HCV Infection in Massachusetts
HCV continues to be one of the highest volume reportable infections reported in Massachusetts. In 2016, there were 7,467 newly reported cases of HCV infection; in 2022, this had decreased by 62% to 2,859 newly reported cases (Figure 1). Diagnosis of current HCV infection requires an RNA test, following an antibody test. HCV testing with reflex to RNA testing (the automatic performing of an RNA test following an antibody-positive result) has increasingly been adopted by commercial and clinical labs and was implemented at the Massachusetts State Public Health Laboratory in July 2018. RNA test results in combination with antibody test results allow for more definitive classification of HCV cases – probable cases either confirmed by positive RNA results or revoked if negative.
This adoption of RNA reflex testing may account for some of the decline in cases observed after July 2018. Analysis of the 2018 and 2019 decline identified no confounding variable(s), supporting the observation that the decreases were not factitious, and potentially were influenced by the prevention efforts and services available for people who use drugs (PWUD), including those funded by DPH, as well as uptake of curative medications preventing onward transmission from those treated.
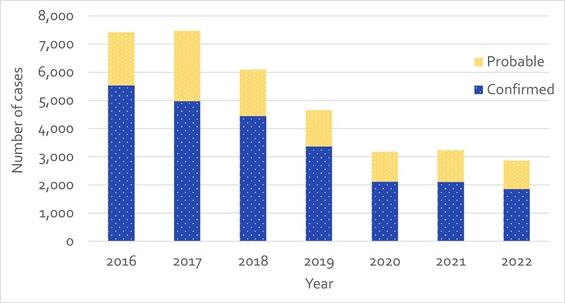
Figure 1 - Number of Confirmed and Probable HCV Cases Reported by Year, Massachusetts, 2016-2022. N=34,890. *Please consider the impact of the COVID-19 pandemic on infectious disease screening, treatment, and surveillance in the interpretation of 2020-2022 data.
The age distribution of HCV cases reported in Massachusetts has shifted over time into younger age groups. A bimodal curve is observed in the age distribution of cases reported in 2022 (Figure 2), in which the highest reported case counts are seen among those between the ages of 25 and 39, with a peak at age 32, representing those most likely to have current injection drug use. This relatively narrow age group alone accounts for 41% of cases reported in 2022.
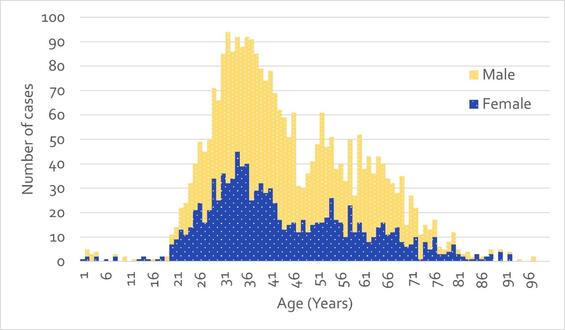
Figure 2 - Number of Confirmed and Probable HCV Cases Reported by Age and Sex, Massachusetts, 2022. N=2,807; 56 cases with missing age and/or sex were excluded from analysis. Cases reported as transgender are not depicted separately due to small numbers.
Surveillance data in Massachusetts show that hepatitis C infection is a statewide issue, affecting rural, suburban, and urban populations, and that acquisition of the infection is overwhelmingly associated with injecting drugs. Of confirmed cases reported between 2016 and 2022 with a known risk history, 95% reported ever having injected drugs (Figure 3).
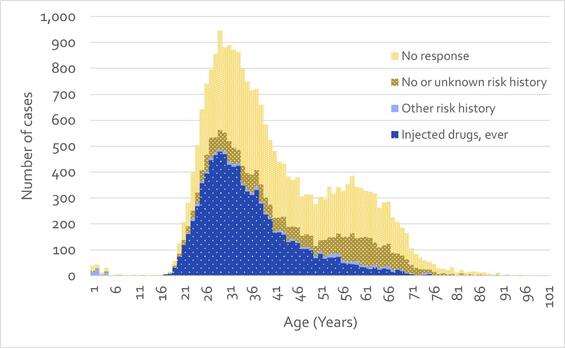
Figure 3 - Number of Confirmed HCV Cases Reported by Age and Risk Factor, Massachusetts, 2016-2022. N=24,304. 70 cases with missing age were excluded from analysis. Note that ‘No or unknown risk history’ indicates that a case report form was received for the case, with ‘No’ or ‘Unknown’ marked for all relevant question in the risk history. ‘No response’ indicates that no case report form was received for the case.
This transmission risk is inextricably linked to the opioid use crisis. Massachusetts overdose death data show that overdose deaths rose to their highest level in 2022, with more than 2,000 deaths each year since 2016, suggesting that injection drug use – and, by extension, the potential for transmission of hepatitis C – has not abated in recent years. We also observe important trends in hepatitis C infection among those with HIV infection. In 2014, 8% of individuals diagnosed with HIV infection were co-infected with hepatitis C. By 2020, this had risen to 19% before falling to 10% in 2022, underscoring the importance of providing services that comprehensively address the wider variety of health issues experienced by people who inject drugs.
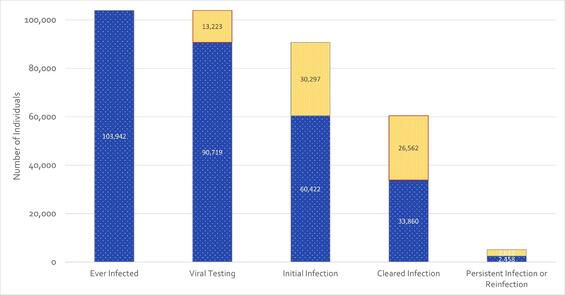
Figure 4 – Laboratory-based Hepatitis C Virus Clearance Cascade, Massachusetts, 2014-2022. N=103,942.
The analytic tool used to monitor prevalence of hepatitis C infection is the clearance cascade (Figure 4). Consistent with CDC methodology, the clearance cascade displays population-level progress through the relevant stages of diagnosis and cure of infection. As shown, 87% of those with any positive test for hepatitis C between 2014 and 2022 had confirmatory RNA testing take place; 67% of those confirmatory tests were positive, indicating active infection at that time. Of the group with initial infection, 56% are known to have cleared infection either spontaneously or by way of treatment. A smaller group, 7% of those known to have cleared, showed evidence of persistent infection or reinfection. The cascade highlights positive progress through relevant clinical stages, but also sheds light on those areas in need of improvement (outlined in red): individuals in need of confirmatory testing, and individuals in need of treatment.
HCV Policy and Legal Environment
The regulatory, legislative, and state policy environment in Massachusetts supports the provision of HCV prevention, screening, and access to treatment, promoting the foundation for elimination.
Harm Reduction
- The sale of syringes has been legal since 2006.
- Syringe Services Programs (SSPs) which provide the majority of harm reduction services in the Commonwealth, have been legal since 1994, with a major legal expansion in 2016.
- The Department completed a feasibility study in December 2023 concerning the establishment of Overdose Prevention Centers (OPCs) in MA. The study recognizes OPCs as an evidence-based, life-saving programs to decrease harm associated with substance use. The study concluded liability protections for all OPC staff and participants must be in place to implement OPC activities in MA. Service components of OPCs, including distribution of sterile supplies and infectious disease testing represent an important and novel tool to advance the elimination of HCV.
Testing Services
- M.G.L.c.111, § 4M1/2 directs primary care providers to offer hepatitis C testing to individuals born between 1945 and 1965.
- A bill filed in the senate, An Act Improving Hepatitis C Screening requires primary care providers to offer HCV testing to anyone over the age of 18 years (repeals the law above).
- 105 CMR 205.000: Minimum Standards Governing Medical Records and The Conduct of Physical Examinations in Correctional Facilities, 205.200(D)(9): addresses mandatory HCV counseling and voluntary testing in correctional facilities.
- 105 CMR 164.000: Licensure of substance abuse treatment programs, 164.043(C)(2); 164.083(B)(10)(11): addresses infectious disease services in licensed treatment programs.
Access to Treatment
- A 2018 settlement agreement with the Commissioner of Massachusetts Department of Correction (C.A.NO. 1:15CV12298NMG) formalized an HCV treatment protocol more consistent with community standards.1
- MassHealth (MA Medicaid Program) recently submitted (10/2023) an amendment to their 1115 MassHealth Demonstration ("Waiver") Amendment Request to CMS to “Provide Pre-Release MassHealth Services to Individuals in Certain Public Institutions” which would cover treatment for justice involved individuals 90 days pre-release. This would include HCV treatment. If approved by CMS (Centers for Medicaid and Medicare Services) implementation of coverage could begin in 2026 or 2027.
- HCV is reportable to both the local board of health and MDPH per Massachusetts regulation 105 CMR 300.000: Reportable Diseases, Surveillance, and Isolation and Quarantine Requirements.
- MassHealth has no restrictions related to liver fibrosis or sobriety on access to DAA treatments, however certain prior authorization requirements remain.
Massachusetts Department of Public Health (MDPH) HCV Response
Bureau of Infectious Disease and Laboratory Sciences (BIDLS)
Massachusetts’ HCV prevention, care, and surveillance efforts are functionally integrated, and integrated across related disease areas. HCV response activities are administered by the MDPH BIDLS. The success of our HCV response relies on highly coordinated activity among multiple BIDLS divisions/offices including the Division of Epidemiology, the Division of Surveillance, Analytics, and Informatics (DSAI), the Office of HIV/AIDS (OHA), the Division of STD Prevention and HIV Surveillance (DSTDP), the Office of the State Epidemiologist and Scientific Support, the State Public Health Laboratory (MA SPHL), the Office of Health Care Planning (OHCP) and with other MDPH Bureaus (such as the Bureau of Substance Addiction Services) and other state agencies (Department of Corrections, Office of Medicaid, Division Of Insurance). To facilitate coordination of HCV response activities, BIDLS convenes a working group led by the BIDLS Director/Assistant Commissioner. The group meets quarterly or more frequently as necessitated by emerging issues, with representatives of all divisions/offices engaged in viral hepatitis activities to promote coordination, support priority setting, and identify emerging issues.
HCV Monitoring and Investigation
HCV infection is reportable to both the local board of health and MDPH. Since 2006, HCV surveillance data have been managed in the Massachusetts Virtual Epidemiologic Network (MAVEN), a secure electronic, web-based system that enables state and local health departments (LHD) to share public health, laboratory, and clinical data efficiently and securely. Ninety-nine percent of Massachusetts’ 351 LHDs are using MAVEN, including the City of Boston. MAVEN captures relevant information on all reportable disease events in a person-based system that allows multiple disease events to be linked to an individual and enables the sharing of demographic information across disease events. MAVEN fully interfaces with the state Electronic Laboratory Reporting (ELR) and Health Information Exchange (HIE) systems. Given the large volume of HCV cases reported to MDPH, active follow-up by MDPH staff and staff at local health departments prioritizes cases indicated to be acute HCV infection.
All laboratories conducting testing on Massachusetts residents are required to report evidence of viral hepatitis infection within 24 hours. All Massachusetts hospital clinical laboratories (72), four large commercial laboratories, and the MA SPHL transmit HCV test results electronically to MAVEN. Since December 2013, all viral hepatitis diagnostic panel results associated with a concurrent positive test results are reportable, including negative HCV RNA results. Massachusetts achieved full ELR reporting from hospital clinical laboratories in April 2015, and over 95% of laboratory reports are received through ELR.
To strengthen and enhance surveillance data and epidemiologic capabilities, BIDLS has implemented Electronic Medical Records Support for Public Health (ESP) in a number of hospitals and health centers. ESP uses specific case detection algorithms comprised of diagnosis codes, laboratory test orders and results, prescriptions, and symptoms to identify reportable conditions based on data elements in electronic health records (EHRs). Data reported electronically to MDPH include patient information; provider and facility information; clinical information including laboratory test results, associated health conditions, symptoms, and medications prescribed; and information about clinical encounters. Data are reported at time of initial case report and updated subsequently as new information is recorded in the medical record, enabling longitudinal monitoring of continuity of care. HCV (acute and chronic) is reported through ESP.
HCV Service Access
BIDLS administers a range of infectious disease prevention, care, and treatment services that integrate HIV, HCV, STI and TB programs. BIDLS manages outbreak response for viral hepatitis in coordination with local health departments and community-based providers. Field epidemiologists provide assistance with notifying sex and drug sharing partners, and for linkage to treatment for individuals identified with both HIV and HCV infection. BIDLS also has a role in supporting workforce development and capacity development for prevention, control, and treatment including facilitating training and education for clinical and non-clinical providers of contracted integrated HIV/HCV/STI and LTBI screening and treatment programs, and development and dissemination of tools to advance HCV screening and treatment. BIDLS supports a range of HCV prevention, linkage, and care coordination services statewide through contracts with medical and non-medical community-based providers. Services include Integrated Testing and Linkage Services (ITLS), Syringe Services Programs (SSPs), Correctional Linkage to Care (CLTC), Short Term Health Navigation (STHN) and navigation services to support engagement in Pre-Exposure Prophylaxis (PrEP) for HIV. These publicly supported prevention, screening, and linkage services, described in more detail below, complement those delivered in healthcare settings, and promote access to these services and advance health equity by addressing social determinants and other barriers to care.
Goals
The Viral Hepatitis National Strategic Plan for the United States: A Roadmap to Elimination (2021-2025)identifies five high-level goals meant to encompass the HCV epidemiologic, service system, and treatment access status of each state. The Massachusetts HCV Elimination Plan uses these high-level goals as a framework and articulates strategies DPH will employ or proposes to employ to advance elimination of HCV in the Commonwealth. Through implementation of the strategies described herein, DPH expects to contribute to reducing new infections, promoting cure of identified infections, and reducing HCV-associated mortality.
Goal 1: Prevent New Hepatitis C Infections
Objective: Facilitate and improve access to HCV prevention and treatment for People Who Use Drugs (PWUD).
- Strategy: Maintain BIDLS rapid response to Local Board of Health Approval of SSPs through existing procurement and funding mechanisms.
Description: Local Boards of Health (LBOH) have the authority to approve a MDPH-implemented SSP in their city or town.2 Rapid identification of a provider, and contract execution or amendments to begin services are essential. Eighty-seven local boards of health have approved an SSP as of January 1, 2024, with 65 operational BIDLS-funded programs in operation.
- Strategy: Strengthen capacity of SSP and other providers serving PWUD to provide testing and linkage to treatment for HCV and related infections.
Description: SSPs support the health of PWUDs by not only providing access to sterile syringes, safe disposal of used syringes, and safer smoking/inhalation supplies, but also providing HIV/HCV/STI testing and linkage services and linkage to substance use disorder (SUD) treatment services. SSPs receive technical assistance to integrate these clinical services.
Objective: Advance additional harm reduction programming as legally allowed.
- Strategy: Monitor legislation and support initiatives with potential impacts on drug use and associated infectious disease transmission.
Description: In 2021, BIDLS, along with the Bureau of Substance Addiction Services (BSAS), expanded access to harm reduction supplies in funded programs by allowing harm reduction programs funded by either Bureau to expand the harm reduction supplies provided to include Fentanyl Test Strips (FTS) and safer consumption supplies for non-injection drug use. These changes were made to address the lethality of fentanyl and the increase in use of stimulants such as cocaine and methamphetamine.3
An Act relative to preventing overdose deaths and increasing access to treatment, is currently pending before the legislature. These companion bills create a framework that would allow oversight and operation of Overdose Prevention Centers (also called Safe Consumption Sites). OPCs have been found to decrease harmful injection behaviors, such as syringe sharing and re-use, resulting in decreased HIV infections. These bills would require DPH to promulgate regulations and establish a licensure process for authorization of a 10-year OPC pilot program as well as set forth legal protections, minimum standards, and required data collection expectations.
Goal 2: Improve Hepatitis C Health Outcomes
Objective: Increase access to HCV treatment.
- Strategy: Facilitate HCV treatment engagement and retention by supporting and promoting interdisciplinary care and supportive navigation services.
Description: Short Term Health Navigation (STHN) as funded through BIDLS is navigation for persons with positive HCV, STI, and/or TB test results and/or who have high risk for HIV, HCV, STI, or TB acquisition and/or transmission. STHN for HCV treatment has a timeframe of 9 months, with the goal of treatment completion and cure.In state fiscal year 2023, 599 individuals were provided with HCV STHN services. BIDLS will continue to work to support the capacity of additional providers, particularly those who work with PWUD, to offer STHN for HCV.
- Strategy: Engage clinical providers to reduce stigma, increase awareness, and stimulate utilization of HCV testing and treatment.
Description: BIDLS supports training opportunities designed to build provider knowledge and skills regarding screening and treatment for HCV. Regional, group-level educational sessions are complemented by “in-service” training/education for individual clinics. In addition, an HCV Community of Practice (CoP) for primary care providers is open to providers statewide and meets quarterly. Each session is co-led by a nurse practitioner and physician.
- Strategy: Support training and education for community service providers.
Description: BIDLS supports training and education for community-based providers using a combination of online, self-paced learning and instructor-led courses. Sessions are designed to improve knowledge and understanding of the epidemiology of HCV, and support implementation of evidence-based prevention and treatment strategies. Faculty for instructor-led courses include clinicians experienced in medical management of individuals with HCV and SUD, as well as frontline staff and managers of prevention, testing, and linkage programs serving PWUD, including SSPs.
Goal 3: Reduce Hepatitis C-related Disparities and Health Inequities
Objective: Support highly targeted services to identify infection, facilitate linkage to treatment, and provide prevention to advance equitable access to services.
- Strategy: Support community-based integrated testing and linkage services.
Description: Integrated Testing and Linkage Services (ITLS) provides recruitment, screening for HCV (and other infectious diseases), provision of test results, and expedited linkage to medical evaluation and treatment. Thirty-eight providers are funded to provide ITLS at a variety of settings across the state: medical facilities (example: community health centers), community-based organizations (example: SSPs), alternative venues (example: drop-in centers), living environments (example: shelters) and mobile van programs (example: vans targeting isolated or hard-to-reach populations).
- Strategy: Support testing and linkage to treatment in county correctional facilities.
Description: Among 34,890 individuals newly reported to MDPH with HCV infection between 2016 and 2022, 3,699 were known to be incarcerated – 1,275 of whom were incarcerated at county Houses of Correction (HOCs). There were 27,111 HCV tests performed at BIDLS-funded sites from 2016-2022, of which 12,641 were confirmed positive. Of the confirmed positives, 3,677 tests were performed at state prisons and 2,392 were performed at the county HOCs. There are 13 county HOCs in the Commonwealth. BIDLS supports testing through outposted services and utilization of the state lab in all but one HOC.
- Strategy: Increase access to services to justice-involved individuals returning to the community.
Description: Correctional Linkage to Care (CLTC) CLTC is a short-term linkage service for people with HCV who are returning to the community to link them to care on release. Individuals with SUD are prioritized for enrollment in the service. CLTC staff meet with eligible individuals three months prior to release and schedule medical, SUD, and other support service appointments for clients to attend upon release. Access to HCV treatment in the county correctional facilities is inconsistent. CLTC is currently available in 10 of the 13 county correctional facilities.
As noted earlier, MassHealth, recently submitted (10/2023) an amendment to their 1115 MassHealth Demonstration Request to CMS to “Provide Pre-Release MassHealth Services to Individuals in Certain Public Institutions” which would cover treatment for justice involved individuals 90 days pre-release. This would include HCV treatment. If approved by CMS (Centers for Medicaid and Medicare Services) implementation of coverage could begin in 2026 or 2027.
Goal 4: Improve Hepatitis C Surveillance and Data Usage
Objective: Analyze epidemiologic impacts of HCV and disseminate findings to inform public health action and to increase public health understanding/awareness.
- Strategy: Regularly compile and publish surveillance reports describing populations affected, risk factors for infection, and changes in the epidemic’s characteristics.
Description: Creation and routine publication of surveillance reports makes data publicly available to stakeholder groups, tracks the epidemic, and informs prevention efforts and policies. Surveillance data will be reviewed and compared over time to assess progress in reducing disease incidence, as estimated by newly reported and suspected acute HCV cases, and prevalence, as estimated by the number of individuals with unresolved positive HCV RNA results. Feedback on the content of surveillance reports will be solicited from partners to ensure utility and relevance.
- Strategy: Publish regular summaries of the HCV care cascade in Massachusetts.
Description: Following CDC standards, the HCV care clearance cascade describes, in succession, individuals who have ever been reported as infected, individuals with follow-up viral testing, individuals infected, individuals cured or cleared, and individuals with persistent infection or reinfection. Care cascade analyses will anchor estimates of the prevalence of individuals living with HCV infection in the state, which can then be monitored over time for reduction in disease burden. The HCV care cascade can be calculated to include the entire population of the state or can be restricted to certain subgroups. Inequities in linkage to care (specifically, segments for which HCV testing or treatment are suboptimal) will be identified by stratifying data across demographic and risk categories, such as race and ethnicity, sex at birth, gender identity, geography, injection drug use status, or incarceration, enabling direct data-to-action efforts.
- Strategy: Routinely review outbreak response protocols for People Who Use Drugs (PWUD).
Description: In support of elimination efforts, outbreak response protocols will be formalized, inclusive of input from relevant partners, and updated on a prospective basis following experiences with their application in specific scenarios.
Objective: Strengthen disease surveillance capacity by advancing data collection and reporting strategies to improve timeliness, accuracy, and completeness of reporting of HCV and related infections, including HIV.
- Strategy: Improve and maintain hepatitis C surveillance infrastructure and processes; monitor data quality and completeness.
Description: Activities to ensure data quality and completeness in support of hepatitis C elimination efforts include collection of negative test results, longitudinal surveillance of cases using ESP, and additional training for case investigators at the local and state level to improve collection of critical demographic information and other variables. The surveillance system will undergo routine maintenance and be adapted as necessary to accommodate new testing methods and changing surveillance practices, such as new variables of interest or updated triage protocols.
Objective: Strengthen traditional surveillance and disease reporting mechanisms with complementary data sources.
- Strategy: Support implementation of tools and strategies to improve utilization of data for population-level monitoring of impact and gaps.
Description: Data sources in addition to the standard electronic laboratory reporting and case investigation enhance surveillance data collection, improve understanding of the epidemic and support elimination efforts. These data sources include use of ESP, case matches with vital records data, utilization of Public Health Data Warehouse, and the All-Payers Claims Database.
Goal 5: Integrate and Coordinate Efforts to Address Hepatitis C among All Partners
Objective: Enhance access to infectious disease programming and services to individuals with substance use disorder (SUD).
- Strategy: Identify technical assistance and capacity building strategies to advance and improve access to infectious disease prevention and care including HCV testing and linkage to care in licensed substance use disorder (SUD) treatment facilities, in conjunction with the Bureau of Substance Addiction Services (BSAS)
Description: The opioid epidemic has led to an increase in SUD and an increase in infectious diseases, particularly HCV. Integrated systems of care with a focus on coordination of recovery services and access to infectious disease screening and treatment are necessary to deliver optimal patient-centered and culturally responsive care and improved health outcomes.
Objective: Maintain collaboration on HCV services with the Department of Correction (DOC).
- Strategy: Support the system of HCV screening and treatment in the DOC.
Description: The DOC screens every new resident for HCV. The DOC treats prisoners with the most serious cases of HCV within 12 months and treats those with less serious cases within 18 months.
BIDLS provides staff support and processes all DOC HCV specimens at the MA State Public Health Laboratory (MSPHL). This support informs surveillance and treatment within the DOC system.
Measuring Progress
Following the standards established by the World Health Organization (WHO) and the CDC, DPH will measure progress toward elimination using the three metrics below4:
- Reduce new HCV infections by 20% by 2025 and 90% by 2030. (Baseline rate: 2.57)
- Reduce new HCV infections among Hispanic/Lantinx individuals by 25% by 2025 and 90% by 2030 (Baseline rate: 2.75)
- Reduce new HCV infections among black/African American individuals by 25% by 2025 and 90% by 2030 (Baseline rate: 1.27)
- Increase HCV rates of cure to 58% by 2025 and 80% by 2030. (Baseline: 56%)
- Reduce rate of HCV-related deaths per 100,000 by 25% by 2025 and 65% by 2030. (Baseline rate: 3.0)
Advancing health equity is a central goal of elimination efforts. DPH will monitor and report on the extent of progress in this regard for each of these objectives for populations where disparities exist, notably PWID/PWUD, Black/African American individuals, Latinx individuals, and individuals living with both HIV and HCV infection. Our goal to strengthen data used to characterize and monitor the epidemic is critical to accountability in this regard. Strategies described in this Plan will contribute to DPH’s capacity to establish baseline measures for priority populations, and to monitor progress toward advancing equity. As data systems/sources evolve and mature, objectives and measurement/reporting strategies will be refined.
Partners: Internal, External, Cross Governmental and Future
The work of HCV elimination will be approached with deep commitment to advancing health equity, and to addressing social determinants of health. The success of efforts to eliminate HCV in Massachusetts depends on continued and deepening partnerships between MDPH and a range of stakeholders including, and importantly, communities impacted by HCV infection, notably people who use drugs. The work of all stakeholder groups complements and informs the activities undertaken to address HCV. BIDLS will continue to work with partners, internal and external to the Department, to support and advance policy and system-level changes that will contribute to elimination of HCV infection in Massachusetts.
External Partners
The MA Viral Hepatitis Advisory Committee (MVHAC) and EndHepCMA Coalition are key community partners supporting MDPH toward eliminating HCV as a public health problem. Since 1999, BIDLS has convened the MVHAC to advise on viral hepatitis-related policy and program issues and includes representation from local public health, healthcare providers experienced in clinical management of hepatitis B and HCV, community health centers, community-based service providers (e.g., syringe service/overdose prevention, testing and linkage), SUD treatment providers, state and local correctional settings, policy advocates, industry, and MassHealth (the state Medicaid) program. The EndHepCMA Coalition is a community-based advocacy group. EndHepCMA is a collaborative effort of clinical providers, advocates, community-based organizations, and consumers working to achieve the elimination of HCV in Massachusetts. This coalition works across stakeholder groups to strengthen HCV-related policies and programs throughout the state through education and community mobilization. EndHepCMA is independent of BIDLS.
In addition to MVHAC and EndHepCMA, BIDLS collaborates with three prevention and care service planning groups: the Massachusetts Integrated Prevention and Care Committee (MIPCC), the Statewide Consumer Advisory Group (SWCAG) and Ending the HIV Epidemic (EHE) Steering Committee. MIPCC is an integrated prevention and care advisory group. The goal of this integrated planning group is to provide advice to BIDLS to implement comprehensive programming for HIV, HCV, and STIs, consistent with BIDLS’ integrated approach to prevention and control of these infections. MIPCC engages in activities required by both the Centers for Disease Control and Prevention (CDC) and the Health Resources and Services Administration (HRSA) for development and implementation of the statewide integrated HIV prevention and care plan. The SWCAGis a designated forum for engagements with individuals living with HIV infection. The SWCAG will meet quarterly to provide advisory input regarding HIV prevention and care, treatment services, and policy strategies. Members of the SWCAG will design and co-facilitate local forums for HIV-positive individuals in urban and rural areas to inform service delivery needs to improve HIV care continuum outcomes, including for individuals co-infected with HIV and HCV. As the group is charged to represent the needs of persons living with HIV, it is expected that HIV-positive members will openly identify as HIV-positive in meetings and community contexts and venues when representing the SWCAG (i.e., presentations, local community convening’s etc.). Since receiving EHE funding in 2020, the EHE Steering Committee has been guiding the EHE process, including production of comprehensive EHE plan for Suffolk County.
Population health advisory groups including the Black Advisory Group, the Latinx Advisory Group, the Gay Men’s Advisory Group, and the Transgender Advisory Group advise BIDLS on ways to improve health outcomes, address health disparities, and focus on best practices and opportunities to improve prevention and care services. The Behavioral Health Advisory Group, a subject matter advisory group, advises BIDLS on topics including the integration of behavioral health responses into infectious disease prevention and care programs.
Internal Partners
Within MDPH, BIDLS collaborates with other Bureaus through senior-level engagements with Bureau Directors. BIDLS works very closely with BSAS regarding services for PWUD and others with SUD. Syringe service programs (SSPs) are fully integrated with overdose education and naloxone distribution (OEND) services, and BSAS and BIDLS coordinate development and monitoring of these services. BIDLS and BSAS collaborate in developing education and training for staff working in agencies serving PWUD, and in development and dissemination of consumer-focused educational materials for PWUD. BIDLS and BSAS are currently focusing on the integration of infectious disease services, notably HIV, HCV, and STI testing and linkage, within SUD treatment setting. Capacities built through these activities will also serve to strengthen Massachusetts’ viral hepatitis response.
Cross Governmental
Outside of MDPH, BIDLS collaborates with the MassHealth Program (the MA Medicaid program) and the Department of Corrections (DOC). MassHealth provides health benefits and help paying for them to qualifying children, families, seniors, and people with disabilities living in Massachusetts. The principal collaboration on HCV focuses on coverage for screening and treatment. The Senior Pharmacy Director in MassHealth’s Office of Clinical Affairs is a member of the MVHAC. The DOC oversees the state prison system, through the management of 15 institutions across the state. Collaboration focuses on screening for HCV and access to treatment while incarcerated.
Future Partners
BIDLS plans to collaborate with the Inter Agency Tribal Partner Work Group and the Institute for New England Native American Studies. The Agency Tribal Partner Work Group brings together state agencies working or seeking to work with Tribal and Native American Communities. The Institute for New England Native American Studies hosts a community advisory board (CAB) for American Indian/Alaska Natives which provides community input.
To ensure community input into HCV elimination strategies, BIDLS will be holding a series of interviews with people with lived experience. Interviews will be conducted with SSP participants who have been diagnosed with HCV but have not received treatment to help identify barriers to treatment.
Supporting the Elimination Plan
The support of partnerships and an enabling environment allow BIDLS to provide community-based services, produce data and relevant epidemiologic and policy analyses, and address certain structural systems to help eliminate HCV infection. This cannot be sustained without assistance. This assistance includes additional funding for services, and to support public health response and response systems, assurances from partners to accomplish initiatives, and policy modifications on the state level to address elimination.
Services
To advance elimination, DPH will require additional resources to fully support existing prevention and harm reduction services, and to expand to appropriately address existing gaps. DPH will need to support expanded SSP and related harm reduction services once implementation has been approved by municipalities. As of 1/1/2024, 25% or 87 out of 351 Massachusetts municipalities have approved SSP, though more approvals are anticipated. Expansion of ITLS services is needed in underserved areas. The period of incarceration represents an exceptional opportunity to provide curative treatment to individuals with HCV infection, thereby advancing elimination. Pilot programming supported currently by BIDLS demonstrates the value of dedicated staff to facilitate screening, and engagement with curative treatment for HCV either during incarceration or at release to the community. Resources to support development of capacity, including dedicated staffing, to coordinate and facilitate infectious disease services within HOCs, and upon release to the community is critically needed. Expansion of HCV STHN to underserved areas will enhance treatment support.
Partners
Request clinical laboratories and health systems implement the CDC recommendation of RNA reflex testing to identify current infection, to facilitate timely entry to curative treatment, and to enable surveillance system to correctly characterize infection.
Encourage health systems and health care providers, including primary care providers, to screen for HCV based on CDC Guidelines for universal screening and to initiate treatment for individuals diagnosed with HCV infection.
Expand continuing medical education for clinical providers to ensure knowledge and understanding HCV infection including: the value of screening; appropriate testing strategies; availability of curative treatment, and treatment regimens that may be administered in primary care; processes for treatment access; and to address stigma and myths associated with drug use that are current barriers to screening and treatment. Training programs are needed for currently practicing clinicians and support staff. Support and investment in this area is needed from professional organizations, clinical training programs, medical schools, and other applicable entities.
Develop system and payment models to integrate HCV screening and treatment in substance use disorder treatment programs, as 95% of confirmed cases report having used drugs. BIDLS, BSAS and MassHealth are currently collaborating on the integration of HCV screening in community based Opioid Treatment Programs (OTP).
Develop and implement education and training for medical staff and of correctional programs and incarcerated individuals that addresses prevention and treatment of hepatitis C and related infections, and which addresses stigma associated with drug use. Education and training for medical staff has been developed by BIDLS clinical staff and provided at one HOC. An educational video on HCV infection and treatment has been developed and released in one HOC, with plans to use at additional HOCs.
Policy
DPH supports routine HCV testing for all individuals over 18 years old, consistent with national screening recommendations. This will help support developing a true picture of HCV in MA, and for individuals with HCV infection it will facilitate entry to curative treatment, thereby also contributing to reduction in transmission DPH also supports routine, periodic testing for individuals at elevated risk, for acquiring HCV infection, notably PWUD. Routine periodic testing among PWUD is essential to early identification of infection which facilitates engagement with curative treatment, thereby interrupting transmission. Such testing, particularly delivered as a component of comprehensive drug user health services, also provides an opportunity for provision of harm reduction and other support services to prevent transmission.
DPH released a feasibility report on OPCs in December 2023.The Department itself completed the study to examine the feasibility of establishing OPCs in MA. The study recognizes OPCs as an evidence-based, life-saving programs to decrease harm associated with substance use. The study concluded liability protections for all OPC staff and participants must be in place to engage in OPC activities in MA. Service components of OPCs, including distribution of sterile supplies and infectious disease testing represent another tool to address the elimination of HCV.
Additional Resources
-
Open PDF file, 970.74 KB, Massachusetts Hepatitis C Elimination Plan (English, PDF 970.74 KB)