- Scientific name: Ambystoma laterale
- Species of Greatest Conservation Need (MA State Wildlife Action Plan)
- Threatened (Bristol and Plymouth counties); Special Concern (remainder of state) (MA Endangered Species Act)

Description
Blue-spotted salamander is a medium-sized salamander with conspicuous markings of randomly distributed, sky-blue spots, blotches, and flecks on a base color of dark gray to black. While the blue markings are abundant over the entire body in juveniles, they tend to be more concentrated along the sides and on the limbs in adults. Adults measure 7.5-13 cm (3-5 in) in total length. The tail is laterally compressed (especially in sexually active males) and is proportionally longer in males than in females. Blue-spotted salamander is in the family of mole salamanders (Ambystomatidae), and so it has distinctively long toes and a stockier build relative to other groups of salamanders in our region.
Larvae have bushy, external gills and a broad caudal fin that extends well onto the back. Young larvae are not easily distinguished from those of other Ambystoma species. Older larvae can still be difficult to identify, but they are generally characterized as brownish with a yellowish lateral stripe, whitish/unpigmented undersides, and a heavily dark-mottled caudal fin.
Unisexual Form
Blue-spotted salamander is a member of an intriguing group of mole salamanders known as the Ambystoma jeffersonianum complex. In Massachusetts, the complex consists of two bisexual species – Jefferson salamander (A. jeffersonianum) and blue-spotted salamander – and a group of unisexual Ambystoma of a hybrid lineage. Unisexual Ambystoma in this complex have variable nuclear genomes consisting of complements of both blue-spotted salamander and Jefferson salamander, and a mitochondrial genome derived from streamside salamander (A. barbouri), a species currently occurring in Kentucky, Ohio, Indiana, Tennessee, and West Virginia. The original species pairing that led to the hybrid unisexual lineage is not yet known, but studies suggest that today’s unisexual Ambystoma and A. barbouri from western Kentucky share a maternal ancestor from ~5 million years ago. The unisexual Ambystoma, whose populations almost always consist entirely of females, co-occur with local populations of genetically pure blue-spotted salamanders or Jefferson salamanders and are able to perpetuate through complicated reproductive mechanisms involving the use of sperm from males of either of those two species. The resulting offspring are unisexuals having varying ploidy levels (usually 3-4 sets of chromosomes, but occasionally 2 or 5) and varying complements of A. jeffersonianum vs. A. laterale nuclear genomes (depending on which of the species is present at a given site, and which reproductive mechanism plays out for a given egg). Unisexuals are not recognized as distinct species or subspecies; rather, they are considered hybrid forms of whatever species with which they are breeding. Across the entire geographic range of the lineage, unisexual Ambystoma are known thus far to breed with 5 different mole salamander species. Contrary to a popular, outdated hypothesis, pure blue-spotted salamanders do not actively breed with pure Jefferson salamanders to produce the unisexual hybrids.
The pure vs. unisexual forms of blue-spotted salamander can often (but not always) be distinguished in the field by size and coloration; adult unisexuals tend to have a gray to gray-brown base color (instead of jet black) and are noticeably larger (usually ≥70 mm [≥2.8 in] snout-vent-length, ≥7 g [≥0.25 oz]) than pure blue-spotted salamanders (frequently ≤60 mm [≤2.4 in], ≤6 g [≤0.21 oz]). In addition, one can assume with very high probability that any male specimen encountered in the field is the pure form.
Similar Species
Jefferson salamander is similar in appearance to blue-spotted salamander, but adult Jefferson salamanders are larger (usually ≥80 mm [≥3.1 in] snout-vent-length and ≥8 g [≥0.28 oz]) than adult blue-spotted salamanders of the pure form (usually ≤70 mm [≤2.8 in] snout-vent-length and ≤7 g [≤0.25 oz]). Furthermore, Jefferson salamanders have a gray or grayish brown base coloration (rather than black) and less prominent markings (smaller, light blue to silvery flecks rather than larger, sky-blue blotches). Larvae, juveniles, and unisexuals are not readily distinguishable between the two species without laboratory analysis. However, populations of Jefferson salamander and blue-spotted salamander in Massachusetts tend not to overlap; the only places where they are known to occur in proximity are in the towns of Sheffield and Granby. Therefore, geographic location is usually a reliable means for distinguishing the species.
Some people confuse the gray (or “lead”) color morph of eastern red-backed salamander (Plethodon cinereus)for blue-spotted salamander. However, eastern red-backed salamander is much leaner in overall appearance. Although it has a rather uniform peppering of minute, light-colored flecks along its lower sides, the pattern is quite inconspicuous relative to the larger, bolder, randomly distributed spots/blotches of blue-spotted salamander. An easy way to tell the two species apart is to examine the toes. They are very short and stubby in eastern red-backed salamander, but long and fingerlike in blue-spotted salamander (even in juveniles). In addition, the tail of eastern red-backed salamander is round in a cross-section, whereas the tail of blue-spotted salamander is laterally compressed.
Life cycle and behavior
As the family name “mole salamander” implies, adult and juvenile blue-spotted salamanders spend most of their time underground or hidden beneath rocks, logs, leaf litter, or other debris. During rainy or otherwise humid nights in warmer months of the year, individuals may occur on the ground surface for purposes of foraging, dispersal, or migration to breeding sites. However, most time is spent under leaf litter, in rodent tunnels, or in other subsurface cavities. Winters are spent below the frost line, presumably in vertical rodent tunnels and cavities associated with root channels, as has been observed in other mole salamanders.
Sometime between late February and early April (depending on the precise timing of winter thaw and warm, nocturnal rains at a given site), adult blue-spotted salamanders emerge from their underground retreats and migrate en masse to their breeding wetlands. Breeding migrations are typically triggered by a steady rain with ambient air temperature holding above 40°F for several hours. If favorable weather conditions have arrived before dusk, peak salamander movement tends to occur between an hour after sunset and midnight. Not all individuals migrate on the same night, and not all are able to complete their journey in a single evening. Therefore, migrations may occur over the course of several nights to a couple of weeks, depending on the timing, duration, and frequency of suitable weather conditions. Individuals residing far from their breeding sites may seek temporary cover in small mammal tunnels or beneath moisture-retaining logs, stones, or other objects at the ground surface between migration opportunities. If nocturnal rains are slow to materialize during the normal migratory period, the salamanders may settle for drizzle or a low fog or even migrate beneath the cover of leaf litter (still moist from snowmelt or ground thaw).
Once in their breeding wetland, blue-spotted salamanders engage in an elaborate courtship like that of Jefferson salamander. Various stages may be repeated or abandoned multiple times when a female is not receptive to a male, or when competing males disrupt or otherwise interfere with one another, but courtship generally proceeds as follows. The male approaches a female, orients his body perpendicular to hers, and nudges her side with his snout several times. He then swims over the female, clasps her body behind her forelegs (with his own), and holds her for several minutes. During that time, the two salamanders may swim about as a clasped pair or just rest on the pool bottom. Eventually, the male (while clasping the female) begins rubbing his chin over her snout in a side-to-side motion and vibrates or rubs his hind limbs along her sides. He then releases the female, moves forward while vibrating his body, and arches and undulates his tail. She follows and noses his cloaca. The male then deposits one to several spermatophores on the bottom substrate of the wetland. The female moves over the spermatophore and picks up its seminal fluid (or even the entire spermatophore) with her cloacal lips, drawing it into her body.
In the pairing of males and females of the pure form of blue-spotted salamander, reproduction proceeds via normal fertilization of the eggs by the sperm obtained from the spermatophore(s) (i.e., syngamy of haploid gametes). However, in the pairing of males with females of the unisexual form, reproduction proceeds via any of several possible mechanisms (collectively termed kleptogenesis) that do not involve traditional syngamy. Most commonly, the unisexual produces unreduced, polyploid ova, and the male’s sperm merely activates embryonic development in the eggs without contributing any genetic material, thereby resulting in offspring that are essentially genetic clones of the unisexual mother. That unisexuals never produce offspring of the pure form is one reason why unisexual Ambystoma are believed to predominate in many local populations.
After mating, a female blue-spotted salamander deposits her eggs either singly or in small clusters contained within a thin, jelly-like coating. The eggs are deposited underwater, and the coating subsequently swells to form a loose, clear, gelatinous matrix. Collectively, a cluster of eggs and their surrounding matrix are called an “egg mass”. Females of the pure form always deposit their eggs singly, whether in isolation or in small groups of 2-5 eggs deposited consecutively, side-by-side (i.e., not within a unifying matrix). Females of the unisexual form, however, deposit eggs singly and in masses. The masses typically contain 5-15 eggs each, though some may contain as few as 2 or as many as 30 eggs. In both forms, eggs are usually attached to submerged vegetation, the twigs of submerged shrubs, or to leaves, twigs, and other detritus on the bottom of the wetland. Eggs may also be attached to moss skirting the crotches of submerged shrubs. blue-spotted salamanders tend to produce up to several hundred mature ova, and so a single individual can account for many eggs (pure and unisexual forms) or multiple egg masses (unisexual form) at a wetland.
A female blue-spotted salamander ovipositing on an oak leaf at the bottom of a vernal pool in Attleboro, Massachusetts. After arching her body and depositing a single egg, she promptly moved back beneath the leaf litter and out of sight.
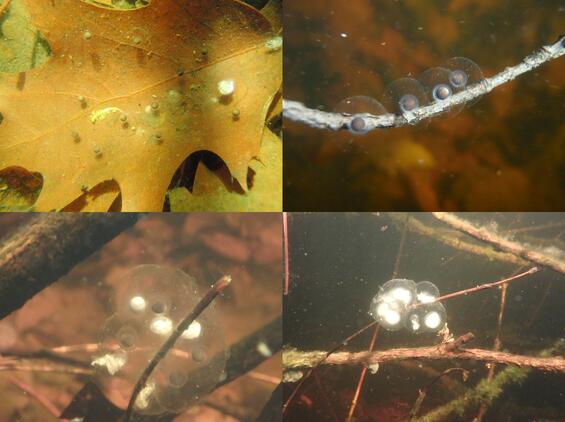
Pure-form blue-spotted salamanders always deposit their eggs singly, sometimes on leaves at the bottom of a wetland (upper left) or along twigs suspended in the water column (upper right). Unisexuals may deposit their eggs singly or in small clusters contained within a clear, gelatinous matrix (lower photos). Egg mortality in unisexual egg masses is common during early stages of embryogenesis, and the decaying egg matter turns conspicuously white.
Hatching occurs in 3-4 weeks, whereupon the bushy-gilled, fully-aquatic larvae spend the next 2-3 months in the wetland. The salamander larvae feed voraciously on zooplankton, insect larvae (e.g., mosquitoes), and other aquatic organisms, increasing in body size and developing front and hind limbs as spring advances into summer. Metamorphosis then occurs in July or August, depending on when the wetland begins to dry, when food resources become limited, or on other factors. At this time, the larvae seek protective cover beneath leaf litter, logs, woody debris, or other objects in saturated portions of the wetland basin while they develop lungs and resorb their gills and caudal fin. Then, the newly transformed metamorphs will wait for an opportunity (typically a nocturnal rain) to leave the basin and disperse into the surrounding forest to begin their lives as terrestrial, juvenile salamanders.
Following dispersal from natal wetlands, juvenile salamanders will reside in the forest, feeding on snails, earthworms, beetles, and other small invertebrates. Upon reaching sexual maturity in approximately 2 years, most individuals will return to their natal wetland to breed, starting the cycle anew. Others will have sought new ground, joining a different subpopulation within a broader metapopulation, or pioneering a new population of their own.
Maximum life expectancy of blue-spotted salamander is unknown, but lifespans of 4-8 years are probably common in most populations, based on studies of this and other mole salamander species. A 5-year study of salamanders at a breeding pond in Massachusetts observed age ranges of 2-8 years (mean 3.7 years) in blue-spotted salamander and 2-10 years (mean 5.2 years) in spotted salamander (Ambystoma maculatum). A monitoring project in Maine documented a blue-spotted salamander (unisexual form) that was at least 12 years old. A study of a spotted salamander population in Quebec, using skeletochronology as an age-estimation technique, observed peak age distributions at 7 years and 15 years, with a maximum observed age of 32 years.
Distribution and abundance
Blue-spotted salamander is largely restricted to glaciated areas of North America. The species ranges from Newfoundland, Quebec, and the Maritime Provinces south to northern New Jersey and west to eastern Iowa, Minnesota, and southeastern Manitoba. Within Massachusetts, blue-spotted salamander is distributed primarily throughout Essex, Middlesex, and eastern Worcester counties. Scattered populations occur in Norfolk, Plymouth, northern Bristol, eastern Hampden, eastern Hampshire, and extreme southern Berkshire counties. As of January 2025, approximately 149 local populations had been documented among 87 towns between 2000 and 2024. Only five populations west of the Connecticut River have been confirmed (all in Sheffield). Populations of blue-spotted salamander in Bristol and Plymouth counties appear to consist exclusively of the genetically pure form, representing a very rare population type in the eastern United States. Based on occurrence records from 2000 to 2024, Massachusetts hosts at least 15 such populations, far more than are known from other states in our region. All populations of blue-spotted salamander elsewhere in Massachusetts are either known or presumed to contain both pure and unisexual individuals, with the latter often predominant.
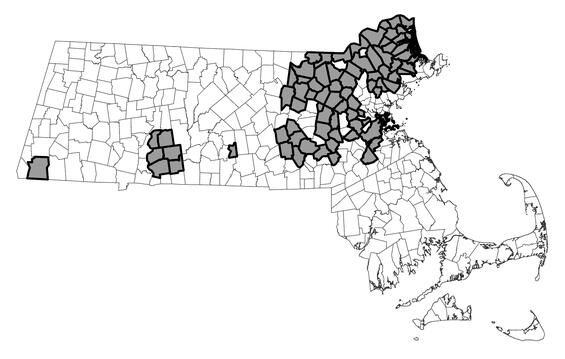
Ambystoma laterale pop.1
Distribution in Massachusetts.
2000-2024
Based on records in the Natural Heritage Database.
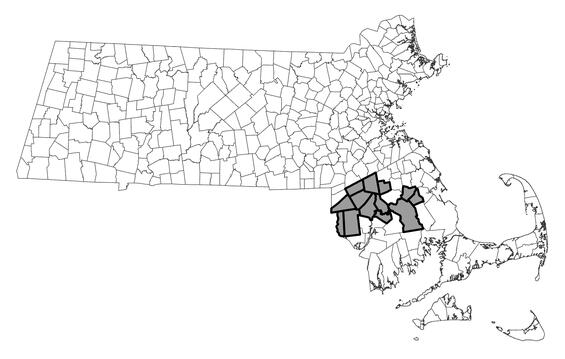
Ambystoma laterale pop.2
Distribution in Massachusetts.
2000-2024
Based on records in the Natural Heritage Database.
Population status
Blue-spotted salamander (including the unisexual form) is legally protected pursuant to the Massachusetts Endangered Species Act (M.G.L. c. 131A) and implementing regulations (321 CMR 10.00); populations in Bristol and Plymouth counties are listed as threatened, whereas populations everywhere else in the state are listed as a species of Special Concern. Biological survey results and anecdotal evidence suggest that declines in population size at the local level and in population abundance at the state level have occurred since the 1990s.
Habitat
Adult and juvenile blue-spotted salamanders inhabit deciduous and mixed deciduous-coniferous forests and woodlands with relatively sandy to loamy soils. In Massachusetts, most populations appear to inhabit lowlands associated with former glacial lakes and glacial stratified deposits. Breeding habitats include marshes and shrub swamps bordering streams and small rivers, maple swamps, isolated shrub swamps, and vernal pools. Although there is considerable variability among the types of wetlands used by blue-spotted salamanders in Massachusetts, some common characteristics include a relatively long hydroperiod, dark water, and moderate to high densities of multi-stemmed shrubs (especially Cephalanthus occidentalis) and/or emergent vegetation (e.g., Typha spp., Carex spp.). Abundant detritus and absence of predatory fish (or presence of refuge from fish) are additional characteristics of typical breeding habitat.

Some common types of wetlands used for breeding by blue-spotted salamanders in Massachusetts include cattail marshes (upper left) and maple swamps (upper right) bordering streams and small rivers, isolated shrub swamps (lower left), and vernal pools (lower right).
In the terrestrial environment, well-developed leaf litter, abundant coarse woody debris, non-compacted soils, predominantly closed-canopy tree cover, and abundant rodent tunnels are trademarks of good-quality microhabitat for blue-spotted salamanders. Although many populations appear to be associated with eskers and other glacial deposits, there is evidence that blue-spotted salamander – unlike other mole salamanders of New England – also inhabits areas with hydric soils. Most adult individuals reside within several hundred meters of their breeding wetland. Generally, the local distribution of mole salamanders around a breeding site may be influenced by habitat integrity, with salamanders residing closer to a wetland (on average) in intact forest but occupying areas farther from the wetland when a forest patch is fragmented (e.g., by development). Variability in the distribution of high-quality microhabitat around a breeding site is also likely to influence the distribution of individual salamanders around the wetland.
As with other mole salamanders, local populations of blue-spotted salamander occur in varying complexity on the landscape. At one end of the spectrum, a resident population is distributed around and dependent upon a single breeding wetland within an isolated patch of forest (i.e., there is no emigration to or immigration from other populations). However, at the other end of the spectrum, multiple subpopulations occur as an interactive network (or “metapopulation”) across an extensive area of forest containing a multitude of breeding wetlands. Each so-called subpopulation consists mainly of resident juvenile and adult salamanders permanently inhabiting the forest area within several hundred meters of the breeding wetland in which they were born and at which they will subsequently breed. However, some individuals from a given subpopulation disperse away from the breeding site, emigrating to a different subpopulation (and its breeding site), recolonizing the area of an extirpated subpopulation, or pioneering an entirely new subpopulation. Hence, the land areas between and among active and prospective breeding sites provide important dispersal habitat for blue-spotted salamander, allowing individuals to move among subpopulations. Generally, upland forest is the preferred dispersal habitat, and the most critical element is that it does not contain major barriers to salamander movement (e.g., vast open areas, extensive vertical structures, roads with high nightly traffic volume). Dispersal habitat is key to the maintenance of metapopulations and the ecological benefits that metapopulation dynamics confer to long-term population viability in the face of environmental and other stressors.
Healthy habitats are vital for supporting native wildlife and plants. Explore habitats and learn about conservation and restoration in Massachusetts.
Threats
Primary threats to blue-spotted salamander in Massachusetts are habitat loss, habitat degradation, habitat isolation, climate change, anthropogenic mortality and disturbance, and disease. These threats may act alone or in combination to cause direct, indirect, and/or cumulative impacts to a given population.
Habitat Loss
The most common types of habitat loss are the clearing of forests and woodlands and the filling (or ditching) of breeding pools for residential, commercial, industrial, mining, or agricultural development. blue-spotted salamanders depend on both upland forest and wetlands to complete their life cycle, and so substantial loss of either habitat at a site can cause a local population extinction. Habitat loss at smaller scales may disrupt metapopulation dynamics, reduce population size, or otherwise weaken long-term population viability.
Habitat Degradation
Habitat degradation has many forms. Roads, railways, and the various other types of development fragment habitat, creating gaps in the forest and impeding or otherwise disrupting salamander migration and/or dispersal. These open areas present increased risks of salamander desiccation and predation, or they increase travel distances and times for individuals that attempt to go around rather than through such a gap or obstacle en route to a destination (e.g., a breeding wetland). Runoff from roads, parking lots, lawns, crop fields, and other areas introduces chemicals (e.g., petroleum, deicing salts, fertilizers, pesticides) and/or sediments to breeding wetlands, potentially disrupting or inhibiting embryonic development or larval growth and survival. Environmental acidification (acute and chronic) threatens amphibian reproduction in aquatic habitats and may even slow or impede growth in terrestrial habitats. Logging operations disrupt forest ecology (e.g., compact soils, reduce leaf litter, introduce or trigger growth of non-native, invasive vegetation) and, when performed carelessly, create avenues for sediment-laden runoff to enter breeding wetlands. Excessive trail densities for recreational activities (hiking, biking, horseback riding, dog-walking) degrade habitat by removing leaf litter, compacting or eroding soils, reducing and/or interrupting rodent tunnels, and facilitating unnatural introduction and accumulation of nutrients. Direct dumping of refuse (tires, batteries, oil filters, paint cans, etc.) and yard waste into breeding wetlands is another common form of habitat degradation.
Habitat Isolation
When habitat loss and fragmentation result in local populations of blue-spotted salamander becoming isolated (i.e., potential for immigration is eliminated), such populations containing the unisexual form are at risk of long-term decline and extinction, due to the hypothetical “Clanton effect”. Under this hypothesis, unisexuals breed successfully (i.e., obtain sperm from males of the pure form) and produce only unisexuals, which are almost always female. Over time, unisexuals become more abundant at the expense of the pure form, and males are produced at increasingly lower rates as unisexuals outcompete pure-form females for sperm. Eventually, existing males are lost to predation and age and ultimately disappear from the population. Without sperm donors, the remaining population is doomed to extinction. For a unisexual-dominated subpopulation to persist, immigration of males and pure-form females from surrounding subpopulations would be needed so that production of male offspring could continue. When immigration becomes an impossibility (e.g., due to habitat isolation), such populations have low probability of long-term persistence. An Ontario study of a Jefferson salamander population containing unisexuals appears to have been documenting such a decline.
In broader landscapes with adequate habitat connectivity, there must be temporal and/or other factors at play to help ensure that not all subpopulations in a metapopulation experience highly skewed unisexual-to-pure-form salamander ratios simultaneously, or else the entire metapopulation would end up on the same track of decline. Genetic sampling work suggests that all Massachusetts populations of blue-spotted salamander outside of Bristol and Plymouth counties contain unisexuals, though at varying degrees of relative abundance. Reasons for those differences are not known, but it is presumed that isolated populations will have lower probabilities of persistence. In metapopulations, “rescue effect” dynamics may be at play, where pure-form individuals recolonize breeding sites of formerly extirpated subpopulations at different points in time and, therefore, each subpopulation in the metapopulation unit is in a different stage of the hypothetical unisexual immigration, production, domination, and extinction cycle. Loss of habitat connectivity and a reduction in the number of connected subpopulations would threaten the benefits of such a safeguard against the Clanton effect and might explain some of the apparent population extirpations observed in Massachusetts during the past several decades.
Climate Change
A 2024 synthesis of climate data, climate modeling, and climate-related research indicates that temperature, total annual precipitation, and frequency of heavy precipitation events are trending upward in the northeastern United States and are expected to continue to do so into the future. A warming and wetting trend might intuitively suggest potential benefits to amphibians, though that might not be true for northern species, like blue-spotted salamander, whose populations in Massachusetts are closer to the southern portion of the species’ geographic range. Furthermore, the timing, frequency, and intensity of precipitation events are important considerations in evaluating the potential impacts of climate change.
Climate data indicate that the Northeast is experiencing wetter summers and falls and drier winters and springs. Such a shift in precipitation patterns threatens the viability of blue-spotted salamander breeding habitat, as some wetlands may begin the breeding period with lower water volumes and – with continued seasonal drought – drawn down to critical levels or even dry prematurely. Reduced water volumes may leave preferred egg-deposition areas dry or exposed, or they may increase larval density and competition, thereby reducing growth rates. Early pool drying results in substantial or complete reproductive failure, as salamander larvae die before they can complete metamorphosis. Paradoxically, an increasing frequency of heavy precipitation events during early summer may threaten blue-spotted salamander larvae in small wetlands, as sudden and large inputs of rainwater may cause spikes in acidification and leaching of metals, potentially leading to mass mortality. Larger wetlands with greater and more stable water volumes are likely to function as climate refugia under the above climate threat scenarios, but shifts in precipitation patterns do threaten the short- and long-term productivity of smaller breeding wetlands (e.g., vernal pools) and wetlands in which water levels are highly sensitive to precipitation (e.g., marshes and swamps bordering streams and small rivers). Climate change could act as a form of habitat degradation for blue-spotted salamander by impacting wetland functions and consequently reducing the stability of smaller populations, weakening metapopulations, and facilitating declines.
Anthropogenic Mortality
Like other amphibians, blue-spotted salamander is susceptible to direct mortality at the hands of Homo sapiens. Blue-spotted salamanders are slow-moving and often need to cross over roads to reach breeding sites and/or disperse into suitable terrestrial habitats. Where populations occur near roads with high nightly traffic volumes, adult and juvenile salamanders are killed by automobiles annually. Increases in automobile traffic over time may tip the scales at some sites and send local populations into continual decline. In extreme cases, perpetually high rates of mortality could result in extinction of a local population.
At a smaller scale, blue-spotted salamanders are vulnerable to mortality via operation of logging equipment during the breeding migration and juvenile dispersal seasons, when individuals are most likely to be beneath cover objects on the forest floor. Other forms of off-road vehicle operation, especially through breeding pools, may kill, injure, or disturb salamanders. Overzealous recreationists, researchers, and educators searching for blue-spotted salamanders beneath cover objects or working in vernal pools disturb microhabitats repeatedly and undoubtedly step on eggs unknowingly.
Disease
Infectious disease has been a significant contributor to global amphibian declines over the past several decades, but impacts in Massachusetts and other parts of New England are not entirely clear.
The amphibian chytrid, Batrachochytrium dendrobatidis (Bd), which is believed to have originated in Asia and spread globally via the pet trade, is a fungal pathogen now prevalent throughout Massachusetts. It is widely blamed for amphibian population declines and extinctions in some parts of the world, but the degree to which it is affecting growth, productivity, and survival in blue-spotted salamanders is not known. Generally, the lethality of Bd infection in amphibians is variable, and some individuals do clear infection, but researchers suspect Bd is a common stressor and contributing source of individual mortality in many amphibian populations of Massachusetts.
The related salamander chytrid, Batrachochytrium salamandrivorans (Bsal) – another Asian fungus that is believed to have spread via the pet trade – has yet to be detected in the wild in North America but has devastated populations of the fire salamander (Salamandra salamandra) in Europe. Ambystomatid salamanders appear to be resistant to Bsal infection, though some species in the genus have been shown to be capable carriers. If (or when) Bsal eventually invades the U.S., direct impacts to blue-spotted salamander are expected to be minimal. However, the eastern newt (Notophthalmus viridescens), which occurs in some blue-spotted salamander breeding wetlands (and likely preys on the salamander’s eggs) in Massachusetts, is highly susceptible to Bsal and could suffer major population declines.
A third major amphibian disease of concern is the group of viruses known as ranaviruses (family Iridoviridae). Like Bd, ranaviruses are believed to have contributed significantly to global amphibian declines. Ranaviruses are established in Massachusetts and are one of the first suspects when people encounter mass mortality of mature amphibian larvae at vernal pools. Multiple common amphibian species in Massachusetts are carriers and effective spreaders of ranaviruses, and so introductions and outbreaks at blue-spotted salamander breeding sites appear to be unavoidable. Sustained or repeated outbreaks at a given breeding site could threaten the local population of blue-spotted salamander with reduced productivity and, therefore, long-term decline.
Conservation
Some prospective conservation measures for blue-spotted salamander in Massachusetts may be grouped into three general categories: inventory and monitoring, management, and research. MassWildlife’s Natural Heritage & Endangered Species Program (NHESP) is the state authority for coordinating and implementing such measures, often in collaboration with other agencies, academic institutions, land trusts, municipal conservation departments, private-sector herpetologists, and others. The NHESP also facilitates participation by the general public.
Inventory and Monitoring
Inventory surveys are essential to discovering undocumented populations or subpopulations of blue-spotted salamander on the Massachusetts landscape and to evaluating the relative robustness of each. Knowledge about population abundance and distribution is prerequisite to understanding the conservation status of the species and to making informed decisions about how and where to invest scarce conservation resources for implementation of management strategies. The NHESP, its collaborators, and the public have made strong gains in documenting populations of blue-spotted salamander and understanding the state distribution of the species over the past several decades, but undoubtedly there are additional populations to be found and evaluated.
Periodic monitoring surveys are necessary to stay informed about population statuses over time. These surveys provide insight about the effectiveness of certain management strategies, and they serve to detect site-specific threats or signs of population decline or extirpation.
Management
At a local scale, sites of known occurrence of blue-spotted salamander should be managed to develop or maintain predominantly mature forest conditions within approximately 1,000 ft of confirmed and potential breeding wetlands. Such management should aim to minimize forest loss/fragmentation, road traffic, recreational trail density, soil compaction, and introduction/growth of invasive, non-native vegetation. Forest type should be maintained as deciduous or mixed deciduous-coniferous. Fallen trees, branches, leaves, and other detritus should be allowed to accumulate on the forest floor. Hydrology of breeding wetlands should not be altered in ways that might reduce hydroperiod within the February through August time period. Breeding wetlands should be protected from chemical pollution, and basin structure should not be altered without special permits from the Massachusetts Division of Fisheries and Wildlife and/or the Department of Environmental Protection. Breeding wetlands should not be filled or used for dumping of yard waste or refuse.
At the landscape scale, land area of mature (or maturing) upland forest between local populations (or subpopulations) of blue-spotted salamander should be maximized to maintain dispersal corridors and, therefore, facilitate genetic exchange among subpopulations and/or recolonization of formerly occupied habitat. Land acquisition and protection efforts for maintaining habitat connectivity should prioritize areas with low road densities and traffic volumes. A land-protection strategy may best serve long-term persistence of local populations where they occupy relatively large, connected areas containing abundant breeding habitats. However, lands supporting small, peripheral, or isolated populations are also worth protecting for maintenance of genetic diversity at the state level.
The long-term viability of Massachusetts populations of blue-spotted salamander having low pure-to-unisexual salamander ratios is not well understood. Therefore, identification and protection of populations with relatively high proportions of pure individuals is considered an important precaution in a changing environment. Biological inventory, research, land protection, and environmental regulation are among the conservation tools that should be utilized to help meet that goal.
Stronger controls are needed to guard against the introduction and spread of amphibian pathogens and infectious disease. For example, possession of and commerce in amphibian species that are listed as Injurious Wildlife by the U.S. Fish and Wildlife Service due to disease concerns should be regulated at both the federal and state levels. In the natural environment, field biologists, researchers, anglers, and others that enter aquatic habitats should adopt and promote appropriate equipment-sanitation procedures between sites, especially when activities span wide geographic areas. A statewide wetland monitoring program that includes non-invasive sampling for pathogens (e.g., Bd, Bsal, ranavirus) and surveillance for amphibian die-offs is needed.
Active management of blue-spotted salamanders and their existing habitats is not a common practice. Using silviculture to favor growth of deciduous broadleaf tree species, and especially mast-producing species, may be a means to improve habitat at some blue-spotted salamander sites by encouraging litter development and higher rodent densities. Conversion of fields to forest may be an effective way to improve habitat connectivity in some situations. Removal and control of non-native trees, shrubs, and vines is assumed to benefit the species by facilitating maintenance or restoration of a native community ecology, especially as it pertains to leaf litter, soil chemistry, and invertebrate prey.
Research
Research is necessary to help fill or improve upon knowledge gaps that might otherwise limit the effectiveness of current practices in conservation planning and management. We will never know everything about Jefferson salamander, but some general topics of conservation research interest include:
- Trends in wetland hydrological regimes, climate change, and salamander breeding phenology;
- Susceptibility of blue-spotted Salamander to amphibian chytrid (Bd) and ranaviruses;
- Trends in environmental acidity at blue-spotted Salamander breeding sites;
- Unisexual and pure-form blue-spotted Salamander dynamics in isolated vs. connected populations;
- Identification of environmental/habitat variables associated with unisexual-dominated vs. pure-form-dominated populations; and
- Design of cost-effective salamander passage structures to enable blue-spotted Salamanders to cross beneath busy roadways.
Community Participation
The general public is encouraged to participate in the conservation of blue-spotted salamander in several ways. For example, observations of blue-spotted salamanders should be reported to the NHESP, as land-protection efforts for the species are dependent on knowing where local populations occur. Collection and submission of data for the certification of vernal pool habitat is another beneficial action, as it will afford certain legal protections to salamander habitat. The Massachusetts community may also provide important information by reporting observations of mass amphibian mortality at vernal pools and other wetlands. All Massachusetts residents are urged to be mindful of the weather, plan ahead, and attempt to minimize the number of times they drive through forested landscapes on rainy nights during early spring and mid- to late summer (i.e., when salamanders are most likely to be on the move and attempting to cross over roadways).
Private landowners in areas of blue-spotted salamander habitat are encouraged to manage their properties in ways that minimize harm to a local population. Some general measures include maximizing the retention of native tree cover and leaf litter, minimizing landscaping, avoiding use of fertilizers and pesticides, and leaving wetlands undisturbed (i.e., keeping dogs out during spring and summer, not removing fallen branches or leaf litter, not dumping yard waste in the wetlands). Landowners may also help to maintain salamander habitat by removing or controlling non-native trees, shrubs, and vines on the property. Another beneficial practice is to maintain vertical guards around window wells and in-ground swimming pools to ensure migrating or dispersing salamanders cannot fall in accidentally. Conversely, landowners are encouraged to identify other potential barriers to salamander movement (e.g., stonewalls lacking holes or gaps, fences flush with the ground) and do what they can to create openings or gaps to allow salamander passage. Making one’s property “salamander friendly” is one of the best ways for residents to participate in conservation of Blue-spotted Salamander and other species.
References
Anderson, K.J. and J.R. Johnson. 2018. The effects of substrate pH on growth and survival of recently metamorphosed marbled salamanders (Ambystoma opacum). Herpetological Conservation and Biology 13:70–79.
Andrews, K.M., J.W. Gibbons, and D.M. Jochimsen. 2008. Ecological effects of roads on amphibians and reptiles: a literature review. Pages 121–143 in J. C. Mitchell, R. E. Jung Brown, and B. Bartholomew, editors. Urban Herpetology. Society for the Study of Amphibians and Reptiles, Salt Lake City, Utah, USA.
Bi, K., and J.P. Bogart. 2010. Time and time again: unisexual salamanders (genus Ambystoma) are the oldest unisexual vertebrates. BMC Evolutionary Biology 10:238–251.
Bogart, J.P. 2019. Unisexual salamanders in the genus Ambystoma. Herpetologica 75:259–267.
Bogart, J.P., and M.W. Klemens. 1997. Hybrids and genetic interactions of mole salamanders (Ambystoma jeffersonianum and A. laterale)(Amphibia: Caudata) in New York and New England. American Museum Novitates 3218:1–78.
Bogart, J.P., and M.W. Klemens. 2008. Additional distributional records of Ambystoma laterale, A. jeffersonianum (Amphibia: Caudata) and their unisexual kleptogens in northeastern North America. American Museum Novitates 3627:1–58.
Bogart, J.P., J.E. Linton, and A. Sandilands. 2017. A population in limbo: unisexual salamanders (genus Ambystoma) decline without sperm-donating species. Herpetological Conservation and Biology 12:41–55.
Charney, N.D., A.T. Ireland, and B.R. Bettencourt. 2014. Mapping genotype distributions in the unisexual Ambystoma complex. Journal of Herpetology 48:210–219.
Clanton, W. 1934. An unusual situation in the salamander Ambystoma jeffersonianum (Green). Occasional Papers of the Museum of Zoology, University of Michigan No. 290:1–14.
Croteau, M.C., N. Hogan, J.C. Gibson, D. Lean, and V.L. Trudeau. 2008. Toxicological threats to amphibians and reptiles in urban environments. Pages 197–209 in J. C. Mitchell, R. E. Jung Brown, and B. Bartholomew, editors. Urban Herpetology. Society for the Study of Amphibians and Reptiles, Salt Lake City, Utah, USA.
deMaynadier, P.G., and J.E. Houlahan. 2008. Conserving vernal pool amphibians in managed forests. Pages 253–280 in A. J. K. Calhoun and P. G. deMaynadier, editors. Science and Conservation of Vernal Pools in Northeastern North America. CRC Press, New York, New York, USA.
Douglas, M. E. and B. L. Monroe, Jr. 1981. A comparative study of topographical orientation in Ambystoma (Amphibia: Caudata). Copeia 1981:460–463.
Faccio, S.D. 2003. Postbreeding emigration and habitat use by Jefferson and spotted salamanders in Vermont. Journal of Herpetology 37:479–489.
Fahrig, L., and T. Rytwinski. 2009. Effects of roads on animal abundance: an empirical review and synthesis. Ecology and Society 14(1):21. <http://www.ecologyandsociety.org/vol14/iss1/art21/>
Fairman, C.M., L.L. Bailey, R.M. Chambers, T.M. Russell, and W.C. Funk. 2013. Species-specific effects of acidity on pond occupancy in Ambystoma salamanders. Journal of Herpetology 47:346–353.
Feuka, A.B., K.E. Hoffmann, M.L. Hunter, Jr., and A.J.K. Calhoun. 2017. Effects of light pollution on habitat selection in post-metamorphic wood frog (Rana sylvatica) and unisexual blue-spotted salamanders (Ambystoma laterale x jeffersonianum). Herpetological Conservation and Biology 12:470–476.
Flageole, S. and R. Leclair, Jr. 1992. Étude démographique d’une population de salamandres (Ambystoma maculatum) à l’aide de la méthode squeletto-chronologique. Canadian Journal of Zoology 70:740–749.
Gamble, L.R., K. McGarigal, and B.W. Compton. 2007. Fidelity and dispersal in the pond-breeding amphibian, Ambystoma opacum: implications for spatio-temporal population dynamics and conservation. Biological Conservation 139:247–257.
Goldspiel, H.B, K.E. Hoffmann, and N.D. Charney. 2023. Ambystoma laterale-jeffersonianum complex (unisexual Ambystoma) longevity. Herpetological Review 54:614–615.
Gray, M.J., E.D. Carter, J. Piovia-Scott, J.P.W. Cusaac, A.C. Peterson, R.D. Whetstone, A. Hertz, A.Y. Muniz-Torres, M.C. Bletz, D.C. Woodhams, J.M. Romansic, W.B. Sutton, W. Sheley, A. Pessier, C.D. McCusker, M.Q. Wilber, and D.L. Miller. 2023. Broad host susceptibility of North American amphibian species to Batrachochytrium salamandrivorans suggests high invasion potential and biodiversity risk. Nature Communications 14:3270. <https://doi.org/10.1038/s41467-023-38979-4>
Gray, M.J. and V.G. Chinchar, editors. 2015. Ranaviruses: lethal pathogens of ectothermic vertebrates. Springer Open. <https://doi.org/10.1007/978-3-319-13755-1_4>
Gray, M.J., D.L. Miller, and J.T. Hoverman. 2009. Ecology and pathology of amphibian ranaviruses. Diseases of Aquatic Organisms 87:243–266.
Green, D.E., K.A. Converse, and A.K. Schrader. 2002. Epizootiology of sixty-four amphibian morbidity and mortality events in the USA, 1996–2001. Annals of the New York Academy of Sciences 969:323–339.
Hoffman, K., M. Hunter, Jr., A.J.K. Calhoun, and J. Bogart. 2018. Post-breeding migration and habitat of unisexual salamanders in Maine, USA. Journal of Herpetology 52:273–281.
Homan, R.N., B.S. Windmiller, and J.M. Reed. 2007. Comparative life histories of two sympatric Ambystoma species at a breeding pond in Massachusetts. Journal of Herpetology 41:401–409.
Karraker, N.E., and J.P. Gibbs. 2011. Road deicing salt irreversibly disrupts osmoregulation of salamander egg clutches. Environmental Pollution 159:833–855.
Kenney, L.P., and M.R. Burne. 2000. A Field Guide to the Animals of Vernal Pools. Massachusetts Natural Heritage & Endangered Species Program, Westborough, Massachusetts, and Vernal Pool Association, Reading, Massachusetts, USA.
Klemens, M.W. 1993. Amphibians and reptiles of Connecticut and adjacent regions. State Geological and Natural History Survey of Connecticut. Bulletin 112.
Kolby, J.E. and P. Daszak. 2016. The emerging amphibian fungal disease, chytridiomycosis: a key example of the global phenomenon of wildlife emerging infectious diseases. Microbiology Spectrum 4(3): EI10-0004-2015. doi:10.1128/microbiolspec. EI10-0004-2015.
Longcore, J.R., J.E. Longcore, A.P. Pessier, and W.A. Halteman. 2007. Chytridiomycosis widespread in anurans of northeastern United States. Journal of Wildlife Management 71:435–444.
Lovett, G.M., T.H. Tear, D.C. Evers, S.E.G. Findlay, B.J. Cosby, J.K. Dunscomb, C.T. Driscoll, and K.C. Weathers. 2009. Effects of air pollution on ecosystems and biological diversity in the eastern United States. Annals of the New York Academy of Sciences 1162:99–135.
Madison, D.M. 1997. The emigration of radio-implanted spotted salamanders, Ambystoma maculatum. Journal of Herpetology 31:542–551.
Martel, A., M. Blooi, C. Adriaensen, P. Van Rooij, W. Beukema, M.C. Fisher, R.A. Farrer, B.R. Schmidt, U. Tobler, K. Goka, K.R. Lips, C. Muletz, K.R. Zamudio, J. Bosch, S. Lötters, E. Wombwell, T.W.J. Garner, A.A. Cunningham, A. Spitzen-van der Sluijs, S. Salvidio, R. Ducatelle, K. Nishikawa, T.T. Nguyen, J.E. Kolby, I. Van Bocxlaer, F. Bossuyt, and F. Pasmans. 2014. Recent introduction of a chytrid fungus endangers Western Palearctic salamanders. Science 346:630–631.
McDonough, C., and P.W.C. Paton. 2007. Salamander dispersal across a forested landscape fragmented by a golf course. Journal of Wildlife Management 71:1163–1169.
Millikin, A.R., D.R. Davis, D.J. Brown, S.K. Woodley, S. Coster, A. Welsh, J.L. Kerby, and J.T. Anderson. 2023. Prevalence of ranavirus in spotted salamander (Ambystoma maculatum) larvae from created vernal pools in West Virginia, USA. Journal of Wildlife Diseases 59:24–36.
Petranka, J.W. 1998. Salamanders of the United States and Canada. Smithsonian Institution Press, Washington, D.C., USA.
Pough, F.H. 1976. Acid precipitation and embryonic mortality of spotted salamanders, Ambystoma maculatum. Science 192:68–70.
Regosin, J.V., B.S. Windmiller, R.N. Homan, and J.M. Reed. 2005. Variation in terrestrial habitat use by four pool-breeding amphibian species. Journal of Wildlife Management 69:1481–1493.
Richards-Hrdlicka, K.L., J.L. Richardson, and L. Mohabir. 2013. First survey for the amphibian chytrid fungus Batrachochytrium dendrobatidis in Connecticut (USA) finds widespread prevalence. Diseases of Aquatic Organisms 102:169–180.
Rittenhouse, T.A.G., and R.D. Semlitsch. 2007. Distribution of amphibians in terrestrial habitat surrounding wetlands. Wetlands 27:153–161.
Rollins-Smith, L.A., and E.H. Le Sage. 2021. Batrachochytrium fungi: stealth invaders in amphibian skin. Current Opinion in Microbiology 61:124–132.
Ryan, K.J. and A.K.J. Calhoun. 2014. Post-breeding habitat use of the rare, pure-diploid blue-spotted salamander (Ambystoma laterale). Journal of Herpetology 48:556–566.
Semlitsch, R.D. 1998. Biological delineation of terrestrial buffer zones for pond-breeding salamanders. Conservation Biology 12:1113–1119.
Skelly, D.K., E.E. Werner, and S.A. Cortwright. 1999. Long-term distributional dynamics of a Michigan amphibian assemblage. Ecology 80:2326–2337.
Snodgrass, J.W., R.E. Casey, J.A. Simon, and K. Gangapura. 2008. Ecotoxicology of amphibians and reptiles in urban environments: an overview of potential exposure routes and bioaccumulation. Pages 177–196 in J. C. Mitchell, R. E. Jung Brown, and B. Bartholomew, editors. Urban Herpetology. Society for the Study of Amphibians and Reptiles, Salt Lake City, Utah, USA.
Staudinger, M.D., A.V. Karmalkar, K. Terwilliger, K. Burgio, A. Lubeck, H. Higgins, T. Rice, T.L. Morelli, A. D'Amato. 2024. A regional synthesis of climate data to inform the 2025 State Wildlife Action Plans in the Northeast U.S. DOI Northeast Climate Adaptation Science Center Cooperator Report. 406 p. <https://doi.org/10.21429/t352-9q86>
Walls, S.C., W.J. Barichivich, and M.E. Brown. 2013. Drought, deluge, and declines: the impact of precipitation extremes on amphibians in a changing climate. Biology 2013:399–418.
Williams, P.K. 1973. Seasonal movements and population dynamics of four sympatric mole salamanders, genus Ambystoma. Dissertation, Indiana University, Bloomington, USA.
Wyman, R.L. 1988. Soil acidity and moisture and the distribution of amphibians in five forests of southcentral New York. Copeia 1988:594–599.
Contact
| Date published: | March 31, 2025 |
|---|
