- Scientific name: Scaphiopus holbrookii
- Species of Greatest Conservation Need (MA State Wildlife Action Plan)
- Threatened (MA Endangered Species Act)
Description
Neither a true frog nor a true toad, eastern spadefoot is a toad-like amphibian in the spadefoot family Scaphiopodidae. Its namesake is a keratinized, sickle-shaped appendage (or “spade”) on each hind foot that facilitates burrowing downward through soil. Eastern spadefoot is short-legged, stout-bodied, blunt-headed, and measures 4.4-8.0 cm (1.7-3.19 in) long. Another unique characteristic is that its pupils, unlike in frogs and toads, are vertically elliptical when constricted, appearing cat-like in bright conditions. Under lower light conditions, the pupils may instead be dilated and resemble a circular starburst. In adults and subadults, upper parts of the body are olive to blackish brown with patterns of yellow, most notably in the shape of a lyre or hourglass on the back. The patterning is normally visible only when the animal is moist or wet, as dust-covered individuals appear drab and non-descript. The skin is fairly smooth but contains small, scattered “warts” (actually glands) that are typically orangish in color. The undersides are dull whitish.
Juveniles resemble smaller versions of adults, except that their base coloration is grayer, the yellowish patterning is much paler, and the orangish glands may seem more prominent or numerous. Recently metamorphosed tadpoles (metamorphs) are generally dark brown to gray and have a pointed, bronze-colored rump.
Eastern spadefoot metamorph found in Southwick, Massachusetts. Note the faint lyre pattern on the back and golden rump.
Tadpoles are rather unique in that the eyes are positioned near the upper front of the head (rather than to the sides), which tapers to a narrow but squared snout. Young tadpoles, when viewed from above, thus appear almost mouselike. As tadpoles age, their base coloration of brown darkens and develops minute, golden flecking. The body becomes somewhat triangular or wedge-shaped, rather than elliptical. The upper musculature of the base of the tailfin turns bronze or golden and is quite distinctive when tadpoles are viewed in the water from above. Mature tadpoles begin to develop a bronze-colored lyre or hourglass pattern on the back, which the animal will retain through metamorphosis and into adulthood. The underside of the tadpole is transparent through all but the latest developmental stages, such that contents of the gut coil are clearly visible.

Some developmental stages of the eastern spadefoot tadpole: very early (upper left), moderately early (upper right), moderately late (lower left), and late (lower right). Note the positioning of the eyes near the top of the head and, as the tadpole ages, the development of gold-colored flecking over the body, bronze to gold coloration in the upper musculature of the tail, and bronze-colored lyre pattern on the back.
Eggs have dark embryos and are clustered in masses of variable shape and size. In each mass, the eggs are contained in a very loose, clear, gelatinous matrix.
Egg masses of eastern spadefoot at a breeding pool in Westfield, Massachusetts.
Life cycle and behavior
Eastern spadefoot is a fossorial species, residing underground in a self-made burrow most of its life. The burrow is not a chambered tunnel with an open entrance, like those constructed by mammals. Rather, in burrowing, a spadefoot essentially buries itself alive. It bores into the ground incrementally, digging with the hind feet. As earth is removed from beneath its hind quarters, the spadefoot shuffles its body to settle backward into the space as the displaced soil is pushed laterally and upward. As the spadefoot continues to work its way downward, the loose, displaced soil that has been forced upward collapses over the animal’s head and body. This process is repeated until the spadefoot reaches a desired depth.
During mid- to late fall in Massachusetts, eastern spadefoots typically burrow 1-2 m (3-6 ft) below the surface so that they may winter safely below the frostline. During warmer months, on the other hand, spadefoots may burrow only several inches deep, presumably to conserve energy over the course of their active season, when they need to resurface periodically. This burrowing behavior, combined with nocturnal habits, enables eastern spadefoot to lead a terrestrial lifestyle without fear of desiccation. Moisture from cool soils surrounding the body may be absorbed through the skin, and hydration is maintained further by avoiding exposure to hot air and direct sun.
The active season for eastern spadefoot in Massachusetts is generally April through October, though activity in late March or early November is possible at some sites in some years, depending on climate and weather conditions. During the active season, spadefoots will emerge from burrows for three reasons: to feed, to change the burrow location, or to breed. Activity above the ground surface is almost exclusively nocturnal.
Emergence for feeding appears to be dependent on ambient air temperature, relative humidity, and sometimes additional factors. Nights with temperature and humidity holding above 18.3 °C (65 °F) and 80%, respectively, are generally considered most suitable for feeding. Individuals that emerge to feed on a given night usually do so less than 90 minutes after sunset, and if favorable weather conditions hold, the spadefoots may stay out all night before returning to burrows around dawn. Eastern spadefoot is a sit-and-wait predator – after emerging, it may move a short distance to a favorable location (often beneath a low branch or other type of cover) and then lie in wait to ambush invertebrate prey that cross its path over the course of the night. Prey items typically include beetles, ants, isopods, crickets, spiders, millipedes, and centipedes. Eastern spadefoots may go days and sometimes weeks between emergences for feeding, probably depending on ground and weather conditions. For example, anecdotal evidence from monitoring surveys in Massachusetts suggests that spadefoots at some sites are relatively inactive during periods of extreme heat and prolonged drought, perhaps because soils harden and/or prey become less available.
A spadefoot may burrow at the same location for many months and even up to several years, but it may also change (or shift) locations periodically. Why spadefoots shift burrow locations is not exactly known; intuitively, it is logical for a spadefoot to continue using an established burrow location where the soil remains loose and easy to dig through, thereby reducing energy expenditure and maximizing time spent feeding. Plausible reasons for a given individual to change its burrow location might include disturbance of the existing burrow site by other wildlife, loss of cover at a preferred feeding location, or local depletion of prey. A study of spadefoot emergence events in a Connecticut population indicates that emergence for so-called “burrow-shifting” tends to occur on rainy nights. Besides the benefit of rain keeping a spadefoot well hydrated as it makes an overland movement, rain also helps to soften unbroken ground, making it easier for an individual to bore through the upper soil layer at a new location.
The breeding season of eastern spadefoot in Massachusetts is typically April through August. Emergence for breeding usually occurs in response to torrential or prolonged rains, most commonly with air temperature holding above 10 °C (50 °F) and often in association with elevated groundwater and/or a sharp drop in barometric pressure. When such conditions align at a given site, spadefoots emerge from the ground by the dozens – or hundreds, depending on size of the local population – and migrate to one or more ephemeral pools that have just filled with water. There, males congregate and erupt into a boisterous chorus of powerful, low-pitched squawking that can be heard a quarter-of-a-mile away or farther. The cacophony attracts females, who proceed to enter the water to mate.
One or multiple males will attempt to mate with each female. In a behavior termed “amplexus”, a successful male will grasp the female from behind, locking his forelimbs around her abdomen and blocking access by competing males. In this position, he fertilizes her eggs as she releases them from her body. The eggs are released in loose masses and are attached to or draped over submerged vegetation, leaf litter, or floating debris in shallow water. The entire breeding event is often executed in a single night, whereupon the adult spadefoots exit the pool and return to their burrows in the uplands. During cooler weather or prolonged rain events, breeding activity may be sustained for up to several days and nights. Breeding is essentially the only time that adult spadefoots may be active and observable during daylight hours.
An amplecting pair of eastern spadefoots at an ephemeral pool in Taunton, Massachusetts. The “spade” appendage, used to facilitate burrowing into soil, is visible on the hind foot of the male in this photo.
Eggs deposited during late spring and summer – when water temperatures are warm – typically hatch in less than 48 hours. In early spring breeding events, however, cool water temperatures may prolong hatching time to several days. In extreme cases, chilled water is believed to result in hatching failure altogether. Eastern spadefoot tadpoles are generally inactive during the first 24-48 hours after hatching, as they attach to submerged vegetation in the immediate vicinity of the remnant egg mass (or to the mass itself) while the mouth and other anatomical structures develop more fully. Within days, however, the tadpoles begin swimming throughout the pool, initially feeding on plankton. Once the labial teeth and mandibles are formed, the tadpoles become omnivorous feeding machines. If water temperatures are warm, tadpoles feed around the clock both day and night, consuming algae, plant matter, and detritus. They even scavenge decaying invertebrate and other animal matter, including dead tadpoles of both their own and other species. Due in large part to their constant feeding behavior, eastern spadefoot tadpoles grow more rapidly than larvae of any other amphibian species native to the Northeast. During the cooler months of April and May, spadefoot tadpoles in Massachusetts typically reach metamorphosis in approximately 5-6 weeks. That time may be reduced to approximately 4 weeks in late spring and under 3 weeks in summer. Following a breeding event at one Massachusetts site in early July, time from egg deposition to tadpole metamorphosis was approximately 18 days.
When mature tadpoles are about to complete metamorphosis, they seek protective cover beneath woody debris, vegetation, moss, or other matter in saturated areas around the pool margin. There, they will resorb their gills and tailfin to become fully terrestrial, air-breathing animals. The metamorphs will remain in the immediate vicinity of the pool for several days to a couple of weeks as they put on additional growth and wait for a good opportunity (e.g., a rainy night) to disperse into the surrounding uplands in search of suitable places to dwell and begin life as juvenile spadefoots.
Most eastern spadefoots will inhabit areas within several hundred meters of where they were born and, upon reaching adulthood, breed at their natal pool. However, some individuals do attempt to disperse away from their natal site. In landscapes with extensive areas of suitable habitat, dispersers may join other subpopulations or pioneer new populations of their own. In isolated habitats, however, dispersers presumably have low survival rates. Individuals that are able to find pockets of reasonably suitable burrowing habitat may live in solitude for the remainder of their lives, or they may attempt a return to their natal site.
In southern states where the core geographic range of eastern spadefoot occurs, time to sexual maturity is generally 1-2 years. In Massachusetts, however, time to maturity appears to be 3-4 years, based on monitoring of populations of known age. The delayed maturity in Massachusetts is probably due to the cooler climate of our region, where the active season is shorter, nightly feeding opportunities are fewer, and growth rates are likely slower. Maximum life expectancy is not known, though one study observed individuals in the wild aged 6 years and, therefore, estimated that some spadefoots live at least 7 years. There is one report of an eastern spadefoot having lived 12 years in captivity.
Because eastern spadefoot has evolved to reproduce in specialized habitats under very specific conditions, populations at a given site do not breed every year. For example, very few Massachusetts populations bred during 2014-2016 or in 2022, due to untimely droughts and/or depressed water tables. However, in a wetter year, some populations can breed multiple times, as was the case in Massachusetts in 2021, 2023, and 2024. Hence, the opportunistic nature of eastern spadefoot breeding behavior indicates a reproductive strategy of explosive breeding with occasional production of large cohorts, coupled with a relatively long lifespan.
Distribution and abundance
Eastern spadefoot ranges from Massachusetts and eastern New York south through the Atlantic Coastal Plain to southern Florida, west through the Gulf Coastal Plain to the eastern Mississippi River Valley in Louisiana, and north through Tennessee and parts of Kentucky into southern Illinois, Indiana, and Ohio. The species is absent from the higher elevations of the Appalachians and the Everglades. Plum Island in Massachusetts is the northern limit of the range. Within Massachusetts, populations of eastern spadefoot occur primarily on Cape Cod; 20 of 33 (61%) populations documented statewide during the period 2000–2024 are on the Cape. Approximately 11 populations are scattered among isolated patches of habitat in Bristol, Dukes, Hampden, Hampshire, and southern Franklin counties. The Plum Island population is the lone representative for Essex County, and a small population in Wayland is the last remaining population known from Middlesex County.
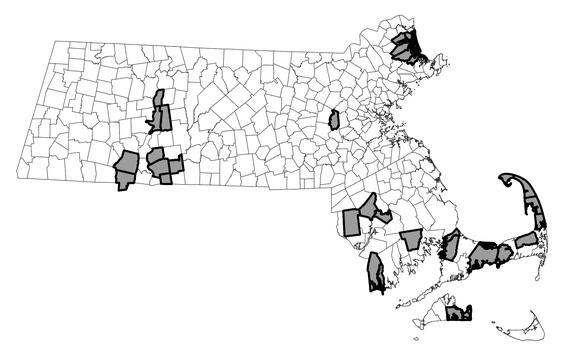
Distribution in Massachusetts.
2000-2024
Based on records in the Natural Heritage Database.
Population status
Eastern spadefoot is legally protected and listed as Threatened pursuant to the Massachusetts Endangered Species Act (M.G.L. c. 131A) and implementing regulations (321 CMR 10.00). Massachusetts is the northern limit of the geographic range of the species, where anecdotal evidence suggests time to sexual maturity is prolonged by 1-2 years. Most local populations outside Cape Cod are isolated, relatively small, and dependent on a single breeding pool. Adult survivorship appears critical to population persistence, especially at sites where reproductive output is low, or reproductive failures are common. Historical records indicate that the species was once more widespread in Massachusetts and has since disappeared from Boston, Cambridge, Concord, Danvers, Framingham, Northampton, Salem, Topsfield, and other localities.
Habitat
The most important upland habitat requirement of eastern spadefoot is a suitable place to burrow. Accordingly, populations are associated with sandy to fine-loamy soils with sparse to moderate vegetation density. In Massachusetts, those habitats tend to occur in coastal dune systems, in barrens, and over glacial stratified deposits within or bordering former glacial lake beds. Although some populations occur almost entirely in forests, most are associated with predominantly open habitats bordering woodlands or containing patches of woody vegetation. Sites with poorly drained and/or stony soils are not suitable for burrowing, nor are areas with high root density (e.g., thatch) or thick leaf litter.
The second critical habitat element for eastern spadefoot is the availability of one or more isolated, ephemeral wetlands within or adjacent to suitable upland habitat. In Massachusetts, eastern spadefoots normally breed in highly ephemeral wetlands that contain standing water for less than 2 months at a time. Such basins support a unique aquatic community containing an abundance of food but fewer predators and competitors than other types of wetlands. Eastern spadefoot breeding pools do not contain fish, and they usually do not support mole salamander (Ambystoma spp.) larvae. Some pools do support other known predators and/or competitors of spadefoot eggs and young spadefoot tadpoles, such as gray treefrog (Dryophytes versicolor), spring peeper (Pseudacris crucifer), wood frog (Lithobates sylvaticus), and toad (Anaxyrus spp.) tadpoles, as well as Dytiscid beetle larvae and some dragonfly species. However, the rapid hatching and growth rate of spadefoot tadpoles may give them a size advantage that enables them to stay a step ahead of the would-be predators, as well as compete well for food resources. Anecdotal evidence from monitoring work in Massachusetts suggests that such an advantage might exist only when spadefoots deposit their eggs as soon as a basin fills with water. If predaceous species establish first, then spadefoots seem to experience complete reproductive failure. That may happen if a pool has filled with water within the reproductive season of the predaceous species but before that of eastern spadefoot, as may happen with dragonfly reproduction in early fall or wood frog and spring peeper reproduction in March. Hence, eastern spadefoot appears to depend on ephemeral pool basins having very specific hydrological regimes that regulate predator communities.
Although breeding habitat must meet specific hydrological regimes, the physical size and structure of eastern spadefoot breeding pools can vary widely. Surface area can be less than 45 m2 (500 sq ft) or greater than 2.6 ha (6.5 ac). Maximum depth can be less than 20 cm (8 in) or exceed 90 cm (36 in). Pools may be beneath a forest canopy or entirely in the open. However, most eastern spadefoot breeding pools in Massachusetts are at least 0.04 ha (0.1 ac) in area, 30-45 cm (12-18 in) deep, and occur in open areas. General types of pools include but are not limited to interdunal swales, dune-system cranberry pools, seasonal woodland ponds, field depressions, old borrow pits, and possibly agricultural ditches.
Healthy habitats are vital for supporting native wildlife and plants. Explore habitats and learn about conservation and restoration in Massachusetts.

Some ephemeral pools used for breeding by eastern spadefoot in Massachusetts: a small cranberry basin in a coastal dune system (upper left); a broad, shallow basin under a wooded canopy (upper right); a large depression in a hayfield (lower left); and an anthropogenic basin at the edge of an agricultural field (lower right).
As with other amphibian species, local populations of eastern spadefoot occur in varying complexity on the landscape. At one end of the spectrum, a resident population is distributed around and dependent upon a single breeding wetland within an isolated patch of suitable upland habitat (i.e., there is no emigration to or immigration from other populations). However, at the other end of the spectrum, multiple subpopulations occur as an interactive network (or metapopulation) across an extensive area of suitable upland habitat containing a multitude of breeding wetlands. Each so-called subpopulation consists mainly of resident juvenile and adult spadefoots permanently inhabiting the area within several hundred meters of the breeding wetland in which they were born and at which they will subsequently breed. However, some individuals from a given subpopulation disperse away from the breeding site, emigrating to a different subpopulation (and its breeding site), recolonizing the area of an extirpated subpopulation, or pioneering an entirely new subpopulation. Hence, the land areas between and among active and prospective breeding sites provide important dispersal habitat for eastern spadefoot, allowing individuals to move among subpopulations. Generally, tracts of sandy or fine-loamy soil are the preferred dispersal habitat, and the most critical element is that such tracts do not contain major barriers to salamander movement (e.g., vast impervious areas, extensive vertical structures, roads with high nightly traffic volume). Dispersal habitat is key to the maintenance of metapopulations and the ecological benefits that metapopulation dynamics confer to long-term population viability in the face of environmental and other stressors.
Threats
Primary threats to eastern spadefoot in Massachusetts are habitat loss, habitat degradation, habitat isolation, climate change, anthropogenic mortality, and disease. These threats may act alone or in combination to cause direct, indirect, and/or cumulative impacts to a given population.
Habitat Loss
The most common type of habitat loss is residential and commercial development in woodlands, fields, and former agricultural areas, as buildings, roads, turf lawns, and other artificial surfaces reduce the land area available to spadefoots for burrowing and feeding. However, the most devastating form of habitat loss might be the filling of ephemeral pool basins, which eliminates reproductive opportunity and is believed to have caused many local population extirpations in Massachusetts. Generally, habitat loss may disrupt metapopulation dynamics, reduce population size, or otherwise weaken long-term population viability.
Habitat Degradation
Habitat degradation typically occurs when development and roads fragment habitat, creating gaps or obstacles that reduce the ability of spadefoots to move through the landscape. Habitats are also degraded when non-native, invasive plant species are allowed to colonize and take over a site, likely impacting prey abundance and diversity.
Habitat Isolation
When habitat loss and fragmentation result in local populations of eastern spadefoot becoming isolated (i.e., the potential for immigration is eliminated), they lose the benefits of metapopulation dynamics. In particular, hypothetical rescue effect – the principle of dispersing individuals recolonizing the site of an extirpated subpopulation, thereby rescuing the subpopulation and bolstering the broader metapopulation – becomes an impossibility. Furthermore, population isolation can lead to declines in genetic diversity within the population. Metapopulations, due to the connectedness and genetic diversity of their subpopulations, are considered more resistant to environmental change and more resilient in response to local extinctions. Therefore, metapopulations are generally more viable than isolated populations over the long term. Habitat isolation is suspected to be a significant factor in historic declines of eastern spadefoot.
Climate Change
A 2024 synthesis of climate data, climate modeling, and climate-related research indicates that temperature, total annual precipitation, and frequency of heavy precipitation events are trending upward in the northeastern United States and are expected to continue to do so into the future. A warming and wetting trend might intuitively suggest potential benefits to amphibians, especially to southern species, like eastern spadefoot, whose populations in Massachusetts are near the northern limits of the species’ geographic range. However, the timing, frequency, and intensity of precipitation events are important considerations in evaluating the potential impacts of climate change.
Climate data indicate that the Northeast is experiencing wetter summers and falls and drier winters and springs. Such a shift in precipitation patterns might threaten the viability of some eastern spadefoot breeding habitats, especially those that are groundwater-influenced and used for breeding primarily in the spring. If water tables are unusually low during spring, larger rain events would be needed to fill spadefoot breeding basins with water, and more frequent rain would be needed to sustain them. Compounding that challenge is that tadpole growth and development are slower during the spring due to cooler water temperatures. In Massachusetts, pools must contain water for a minimum of approximately 5 weeks for tadpoles hatched in April or early May to reach metamorphosis. Therefore, a drier spring climate threatens some populations with greater reproductive failure and – if sustained across several or more consecutive years – could cause precipitous declines.
Conversely, bigger and more frequent rain events during the summer months will cause surface flooding more frequently at basins that are fed primarily by rainwater and surface runoff. Spadefoot tadpole growth and development are very rapid during late June through mid-August, with tadpoles requiring pooled water for only 2-3 weeks during that period to reach metamorphosis. Therefore, an increase in the frequency of summer storms is likely to benefit some spadefoot populations.
Beyond changes in precipitation patterns, a major concern about climate change and spadefoot conservation in Massachusetts is the anticipated effects of a rising global temperature, rising sea level, and increased frequency of tropical storms and hurricanes in the Northeast. The most robust populations of eastern spadefoot in New England are concentrated in just several coastal dune systems of Cape Cod and the South Coast region. They could be vulnerable to massive storm events that cause destructive storm surge and flooding of spadefoot habitat with sea water.
Anthropogenic Mortality
Like other amphibians, eastern spadefoot is susceptible to direct mortality at the hands of Homo sapiens. The primary source of anthropogenic mortality in Massachusetts is likely automobiles. Most inland populations and a number of others on Cape Cod occur in residential areas, where spadefoots must cross over roads during breeding migrations and dispersal events. Spadefoots are relatively slow-moving animals, and so they are seldom able to evade oncoming vehicles. Monitoring work in Massachusetts has documented road-killed spadefoots with regularity.
Undoubtedly, a second source of anthropogenic mortality is construction development. Spadefoots and developers prefer the same kind of habitat (i.e., sandy, well-drained soils), and protective regulatory frameworks are limited. Since spadefoots are always burrowed in the ground during the day, escape from excavators, bulldozers, and other earth-moving machines is virtually impossible.
Adult survivorship is important to long-term population viability and, unfortunately, most Massachusetts populations of eastern spadefoot are subject to unnatural mortality from automobiles and construction equipment. Where road densities, traffic volumes, and construction work are on the rise, eastern spadefoot populations are threatened with reduced survival and productivity and are likely to decline.
Disease
Infectious disease has been a significant contributor to global amphibian declines over the past several decades, but impacts in Massachusetts and other parts of New England are not entirely clear.
The “amphibian chytrid” Batrachochytrium dendrobatidis (Bd), which is believed to have originated in Asia and spread globally via the pet trade, is a fungal pathogen now prevalent throughout Massachusetts. It is widely blamed for amphibian population declines and extinctions in some parts of the world, but the degree to which it is affecting growth, productivity, and survival in eastern spadefoots is not known. Generally, the lethality of Bd infection in amphibians is variable, and individuals do often clear infection, but researchers suspect Bd is a common stressor and contributing source of individual mortality in many amphibian populations of Massachusetts. One study suggests that exposure can at least reduce growth and feeding rates in juveniles of some anuran species.
Another major amphibian disease of concern is the group of viruses known as ranaviruses (family Iridoviridae). Like Bd, ranaviruses are believed to have contributed significantly to global amphibian declines. Ranaviruses are established in Massachusetts and are one of the first suspects when people encounter mass mortality of mature amphibian larvae. Multiple common amphibian species in Massachusetts are carriers and effective spreaders of ranaviruses, and so introductions and outbreaks at eastern spadefoot breeding sites are probably unavoidable. Eastern spadefoot is considered susceptible to ranaviruses, with both infection and mass-mortality events observed in the wild. Sustained or repeated outbreaks at a given breeding site could threaten its local population of eastern spadefoot with reduced productivity and, therefore, long-term decline.
Of particular concern with infectious amphibian disease is the potential for introduction and spread of novel strains via the commercial pet trade. Release of unwanted pets is a potential pathway for foreign strains of Bd or ranaviruses to enter the Massachusetts environment and infect native amphibian populations, many of which are already dealing with other stressors.
Conservation
Some prospective conservation measures for eastern spadefoot in Massachusetts may be grouped into three general categories: inventory and monitoring, management, and research. MassWildlife’s Natural Heritage & Endangered Species Program (NHESP) is the state authority for coordinating and implementing such measures, often in collaboration with other agencies, academic institutions, land trusts, municipal conservation departments, private-sector herpetologists, and others. The NHESP also facilitates participation by the general public.
Inventory and Monitoring
Inventory surveys are essential to discovering undocumented populations or subpopulations of eastern spadefoot on the Massachusetts landscape and to evaluating the relative robustness of each. Knowledge about population abundance and distribution is prerequisite to understanding the conservation status of the species and to making informed decisions about how and where to invest scarce conservation resources. The NHESP, its collaborators, and the public have made strong gains in documenting populations of eastern spadefoot and understanding the state distribution of the species over the past several decades, but undoubtedly there are additional populations to be found and evaluated.
Periodic monitoring surveys are necessary to stay informed about population statuses over time. These surveys provide insight about the effectiveness of certain management strategies, and they serve to detect site-specific threats or signs of population decline or extirpation. The NHESP and its collaborators initiated an integrated monitoring and management program in 2016, which has been invaluable to learning more about spadefoot ecology in southern New England, developing habitat management prescriptions, experimenting with population introductions, and measuring outcomes.
Management
At a local scale, eastern spadefoot sites should be managed to maximize suitable burrowing habitat and favorable breeding habitat. Usually, that entails simply not altering existing habitat. However, at degraded or impaired sites, direct management actions can be taken to restore or improve conditions. Examples include removal and control of non-native vegetation, removal of barriers to spadefoot movement, restoration of breeding-pool basin profiles (e.g., if they have been filled), and conversion of turf, thatch, or any impervious surface to sparsely vegetated, natural ground. In some cases, removing large shade trees over pool basins may help to improve water temperature and reduce early spring water losses to evapotranspiration. Road closures during anticipated breeding events would be helpful in reducing annual adult mortality.
Prior to habitat restoration, this eastern spadefoot breeding pool in a residential neighborhood of Wayland, Massachusetts used to be overgrown with a dense thicket of the non-native, invasive shrub Glossy Buckthorn (Frangula alnus).
At the landscape scale, extensive dune systems at coastal sites and mosaics of woodlands, shrublands, and fields overlying sandy or fine-loamy soils at inland sites should be maintained to preserve dispersal corridors and maximize opportunity for metapopulation function. Land acquisition and protection efforts for maintaining habitat connectivity should prioritize areas with low road and development densities. A land-protection strategy may best serve long-term persistence of local populations and preservation of metapopulation dynamics where eastern spadefoots occupy relatively large, connected areas containing suitable upland habitat and a multitude of ephemeral wetlands. However, lands supporting small, peripheral, or isolated populations are also worth protecting for maintenance of genetic diversity at the state level. Where populations are dependent on a single breeding pool, protection of the pool basin is paramount.
Stronger controls are needed to guard against the introduction and spread of amphibian pathogens and infectious disease. For example, possession of and commerce in amphibian species that are listed as Injurious Wildlife by the U.S. Fish and Wildlife Service due to disease concerns should be regulated at both the federal and state levels. In the natural environment, field biologists, researchers, anglers, and others that enter aquatic habitats should adopt and promote appropriate equipment-sanitation procedures between sites, especially when activities span wide geographic areas. A statewide wetland monitoring program that includes non-invasive sampling for pathogens (e.g., Bd, ranavirus) and surveillance for amphibian die-offs is needed.
Research
Research is necessary to help fill or improve upon knowledge gaps that might otherwise limit the effectiveness of current practices in conservation planning and management. There is also a need to measure known and suspected threats to eastern spadefoot in Massachusetts so that perceived population declines might be better understood. Some general topics of conservation research interest include:
- Trends in breeding basin hydrological regimes, climate change, and spadefoot reproductive success;
- Prevalence of eastern spadefoot mass mortality events caused by ranaviruses;
- Impacts of amphibian chytrid (Bd) to eastern spadefoot growth and survival;
- Identification of genetically distinct or unusual populations in Massachusetts at the state and regional level; and
- Design of cost-effective, artificial pools that exhibit suitable hydrological function for spadefoot reproduction.
Community Participation
The general public is encouraged to participate in the conservation of eastern spadefoot in several ways. For example, observations of eastern spadefoot should be reported to the NHESP, as status assessment and potential land-protection efforts depend on knowing where local populations occur. Collection and submission of data for the certification of vernal pool habitat in the vicinity of eastern spadefoot populations is a potentially beneficial action, as such wetlands are occasionally used by the species and will be afforded certain legal protections if certified. The Massachusetts community may also provide important information by reporting observations of mass amphibian mortality at vernal pools and other wetlands. Residents in spadefoot neighborhoods are urged to be mindful of the weather, plan ahead, and attempt to minimize the number of times they drive at night during and shortly after heavy rains in spring and summer (i.e., when spadefoots are most likely to be on the move and attempting to cross over roadways).
Reporting observations of eastern spadefoot to the NHESP helps to ensure that the latest occurrence information is being used in conservation management decisions.
Private landowners in areas of eastern spadefoot habitat are encouraged to manage their properties in ways that minimize harm to a local population and maximize available habitat or microhabitat. Some general measures include avoiding use of fertilizers and pesticides, replacing turf lawn with native grasses at low stem densities (patchiness and exposure of bare ground are ideal), and minimizing obstacles to spadefoot movement (e.g., fences flush with the ground, stone walls lacking gaps or spaces). Avoid filling or regrading extensive, low areas that fill with water after storms. If mulching flower gardens or other landscaped areas, use fine material in a thin layer so that spadefoots are able to burrow through it. Making one’s property spadefoot friendly is one of the best ways for residents to participate in conservation of eastern spadefoot and other species.
References
Andrews, K.M., J.W. Gibbons, and D.M. Jochimsen. 2008. Ecological effects of roads on amphibians and reptiles: a literature review. Pages 121–143 in J. C. Mitchell, R. E. Jung Brown, and B. Bartholomew, editors. Urban Herpetology. Society for the Study of Amphibians and Reptiles, Salt Lake City, Utah, USA.
Conant, R. and J.T. Collins. 1998. A field guide to reptiles and amphibians: eastern and central North America. Third edition. Houghton Mifflin, Boston, Massachusetts and New York, New York, USA.
Fahrig, L., and T. Rytwinski. 2009. Effects of roads on animal abundance: an empirical review and synthesis. Ecology and Society 14(1):21. <http://www.ecologyandsociety.org/vol14/iss1/art21/>
Goldberg, S.R. 2018. Notes on reproduction of eastern spadefoot toads, Scaphiopus holbrookii (Anura: Scaphiopodidae). Bulletin of the Chicago Herpetological Society 53:63–65.
Gray, M.J. and V.G. Chinchar, editors. 2015. Ranaviruses: lethal pathogens of ectothermic vertebrates. Springer Open. <https://doi.org/10.1007/978-3-319-13755-1_4>
Gray, M.J., D.L. Miller, and J.T. Hoverman. 2009. Ecology and pathology of amphibian ranaviruses. Diseases of Aquatic Organisms 87:243–266.
Greenberg, C.H. and G.W. Tanner. 2004. Breeding pond selection and movement patterns by eastern spadefoot toads (Scaphiopus holbrookii) in relation to weather and edaphic conditions. Journal of Herpetology 38:569–577.
Greenberg, C.H. and G.W. Tanner. 2005. Spatial and temporal ecology of eastern spadefoot toads on a Florida landscape. Herpetologica 61:20–28.
Hansen, K.L. 1958. Breeding patter of the eastern spadefoot toad. Herpetologica 14:57–67.
Jansen, K.P., A.P. Summers, and P.R. Delis. 2001. Spadefoot toads (Scaphiopus holbrookii holbrookii) in an urban landscape: effects of nonnatural substrates on burrowing in adults and juveniles. Journal of Herpetology 35:141–145.
Kirschman, L.J., J.G. Palis, K.A. Fritz, K. Althoff, and R.W. Warne. 2017. Two ranavirus-associated mass-mortality events among larval amphibians in Illinois, USA. Herpetological Review 48:779–782.
Klemens, M.W. 1993. Amphibians and reptiles of Connecticut and adjacent regions. State Geological and Natural History Survey of Connecticut. Bulletin 112.
Klemens, M.W., H.J. Gruner, D.P. Quinn, and E.R. Davison. 2021. Conservation of amphibians and reptiles in Connecticut. Department of Energy and Environmental Protection, Hartford, Connecticut, USA.
Kolby, J.E. and P. Daszak. 2016. The emerging amphibian fungal disease, chytridiomycosis: a key example of the global phenomenon of wildlife emerging infectious diseases. Microbiology Spectrum 4(3): EI10-0004-2015. doi:10.1128/microbiolspec.EI10-0004-2015.
Lazelle, J.D., Jr. 1976. This broken archipelago. Quadrangle/The New York Times Book Company, New York, New York, USA.
Longcore, J.R., J.E. Longcore, A.P. Pessier, and W.A. Halteman. 2007. Chytridiomycosis widespread in anurans of northeastern United States. Journal of Wildlife Management 71:435–444.
McQuigg, J. L., K. Kissner, and M.D. Boone. 2023. Exposure to amphibian chytrid fungus alters terrestrial growth and feeding rate in metamorphic amphibians. Journal of Herpetology 57:36–41.
Pearson, P.G. 1955. Population ecology of the spadefoot toad, Scaphiopus h. holbrookii (Harlan). Ecological Monographs 25:233–267.
Pearson, P.G. 1957. Further notes on the population ecology of the spadefoot toad. Ecology 38:580–586.
Picco, A.M., and J.P. Collins. 2008. Amphibian commerce as a likely source of pathogen pollution. Conservation Biology 22:1582–1589.
Raithel, C.J. 2019. Amphibians of Rhode Island. Rhode Island Division of Fish and Wildlife, West Kingston, USA.
Richards-Hrdlicka, K.L., J.L. Richardson, and L. Mohabir. 2013. First survey for the amphibian chytrid fungus Batrachochytrium dendrobatidis in Connecticut (USA) finds widespread prevalence. Diseases of Aquatic Organisms 102:169–180.
Richmond, N.D. 1947. Life history of Scaphiopus holbrookii holbrookii (Harlan) part I: larval development and behavior. Ecology 28:53–67.
Rollins-Smith, L.A., and E.H. Le Sage. 2021. Batrachochytrium fungi: stealth invaders in amphibian skin. Current Opinion in Microbiology 61:124–132.
Ryan, K.J., A.J.K. Calhoun, B.C. Timm, and J.D. Zydlewski. 2015. Monitoring eastern spadefoot (Scaphiopus holbrookii) response to weather with the use of a passive integrated transponder (PIT) system. Journal of Herpetology 49:257–263.
Skelly, D.K., E.E. Werner, and S.A. Cortwright. 1999. Long-term distributional dynamics of a Michigan amphibian assemblage. Ecology 80:2326–2337.
Snider, A.T. and J.K. Bowler. 1992. Longevity of reptiles and amphibians in North American collections. Second edition. Society for the Study of Amphibians and Reptiles, Herpetological Circular No. 21.
Staudinger, M.D., A.V. Karmalkar, K. Terwilliger, K. Burgio, A. Lubeck, H. Higgins, T. Rice, T.L. Morelli, A. D'Amato. 2024. A regional synthesis of climate data to inform the 2025 State Wildlife Action Plans in the Northeast U.S. DOI Northeast Climate Adaptation Science Center Cooperator Report. 406 p. <https://doi.org/10.21429/t352-9q86>
Sutherland, R.W., P.R. Dunning, and W.M. Baker. 2010. Amphibian encounter rates on roads with different amounts of traffic and urbanization. Conservation Biology 24:1626–1635.
Tennessen, J.B., S.E. Parks, and T. Langkilde. 2014. Traffic noise causes physiological stress and impairs breeding migration behavior in frogs. Conservation Physiology 2(1). doi:10.1093/conphys/cou032. <http://conphys.oxfordjournals.org/content/2/1/cou032.full>
Timm, B.C. and K. McGarigal. 2010. The diets of subadult Fowler’s toads (Bufo fowleri) and eastern spadefoot toads (Scaphiopus h. holbrookii) at Cape Cod National Seashore, USA. Herpetological Review 41:154–156.
Timm, B.C., K. McGarigal, and R.P. Cook. 2014. Upland movement patterns and habitat selection of adult eastern spadefoots (Scaphiopus holbrookii) at Cape Cod National Seashore. Journal of Herpetology 48:84–97.
Walls, S.C., W.J. Barichivich, and M.E. Brown. 2013. Drought, deluge, and declines: the impact of precipitation extremes on amphibians in a changing climate. Biology 2013:399–418.
Contact
| Date published: | April 14, 2025 |
|---|