- Scientific name: Platanthera hookeri
- Species of Greatest Conservation Need (MA State Wildlife Action Plan)
- Endangered (MA Endangered Species Act)
Description

Hooker's orchid (Platanthera hookeri)
Hooker’s orchid has two oval leaves, 6-125 cm (2.4-49 in) and up to 10.5 cm (4.1 in) wide, that lie almost flat on the ground. The plant will often only be found in the vegetative condition, but on some years will send up a leafless flowering stalk grows to 45 cm (18 in) in height with the flowering portion occupying the upper half of the stalk. Anywhere from 6 to 27 up-turned flowers are closely attached to the stalk. These are green to yellowish-green, with a long, 13-25 mm (0.5-1 in) spur and a lip that curves upward (Smith 2012). When it flowers, Hooker’s orchid can bloom any time from mid to late May through the first two weeks of July. It was named after an English botanist, Sir William Jackson Hooker (1785 to 1865).

Hooker's orchid (Platanthera hookeri)

Hooker's orchid (Platanthera hookeri)
Life cycle and behavior
This is a perennial species with an interesting behavioral characteristic in the genus of plants. The tuber-like roots will fully replace themselves every year giving the plant a long lifespan confirmed in one case to be at least 28 years and estimated to be up to 40 years (Smith 2012, Reddoch & Reddoch 2007).
Population status
In Massachusetts, Hooker’s orchid is known historically from Berkshire, Franklin, Hampshire, Hampden, Worcester, Middlesex, Suffolk and Norfolk Counties. Current records are only from western Franklin County. The most recent observation was in 2007 where plants were not in flower but were in the exact same location, with same number of plants, as previously observed. A potential observation was made in 2013, but the plants were not in bloom so could not be confirmed. (There are two other species with essential identical vegetative morphology with this species.) Platanthera hookeri has decreased substantially within Massachusetts. In a 3000-hour fieldwork survey of all 26 towns in Franklin County, where Hooker’s orchid was previously known from 9 towns, the species was not found at all (Bertin et al 2020). The species was not found in any of the towns in the extensive survey of Worcester County, where it previously was known from 7 towns (Bertin and Rawinski 2012). Searcy (2008) did not find any plants in her extensive survey of the Mount Holyoke Range. Finally, Jenkins et al. (2008) did not relocate any plants from Petersham, MA.
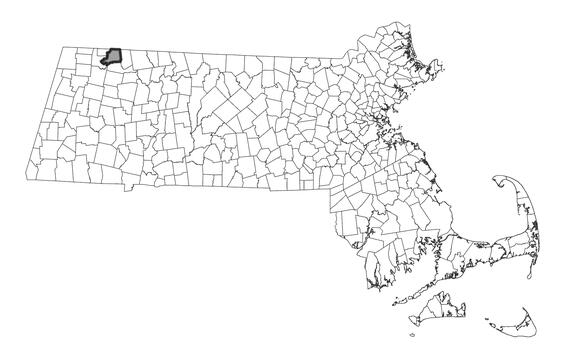
Distribution in Massachusetts. 1999-2024. Based on records in the Natural Heritage Database.
Distribution and abundance
The species ranges from Newfoundland west to Manitoba, south New Jersey. The species has declined dramatically across its range since the early to mid-1900s as documented by Reddoch and Reddoch (2007). Current distribution is shown in the iNaturalist map image of the 490 research grade observations with the earliest records in 1978 and the vast majority of records more recent than 2005 (iNaturalist 2025). Orchids attract a great deal of attention on iNaturalist, as elsewhere, so these maps are considered to be quite accurate.
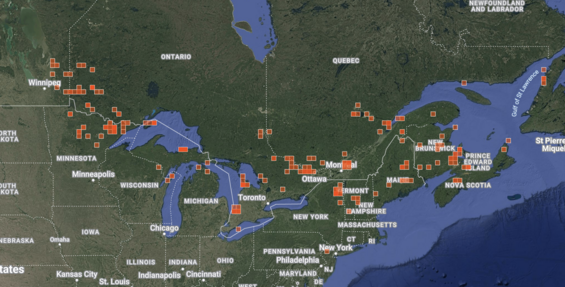
Full range extent of Platanthera hookeri as shown by research grade observations on iNaturalist accessed 26 March 2025.
Habitat
Hooker’s orchid has been found in a variety of habitats but is most frequently observed in secondary growth forests with rich soils. Associated species include sugar maple, white birch, white pine, eastern hemlock, mountain laurel, American hazelnut, wild sarsaparilla, Christmas fern, witch hazel, sessile bellwort, Canada mayflower, violets, southern beech-fern, and maidenhair fern.
Healthy habitats are vital for supporting native wildlife and plants. Explore habitats and learn about conservation and restoration in Massachusetts.
Threats
A study by Reddoch and Reddoch in 2007 of the population dynamics of this species found very little seed set, indicating a lack of pollinator visits, possibly due to shading by canopy closure, herbivory by deer, and acid deposition as the primary causes of the orchid decline. Acid deposition may be affecting the orchids directly, or it may be impacting their associated mycorrhizal fungi.
This species occurs far to the north in North America and in the United States, almost all occurrences occur north of an imaginary line between Minneapolis and Boston. As a species of more northerly distribution, climate warming is a serious threat. The past 130 years have seen a warming of 1.4 degrees C, (2.5 degrees F) in the Northeast United States (Staudinger et al. 2024). Northern species can be expected to move much further north in an attempt to occupy a habitat within their evolved climate envelope. Temperature and seasonality changes also disrupt synchronization of pollinators to plant flowering.
The dust-like seeds of orchids rely on mycorrhizae for its seed germination, protocorm formation and growth into a seedling. Therefore, it is quite likely that changes in species composition and abundance of mycorrhizae will strongly affect orchids over time. Causes of orchid decline could include, but are not limited to, deer herbivory (Knapp and Wiegand 2014), earthworms (McCormick et at. 2013), lack of disturbance (Sheviak 1990), nitrogen deposition (Figura et al. 2020, Whalen et al. 2018), invasive species such as garlic mustard (Anthony et al. 2017), and canopy closure (Brumback et al. 2011, Whigham et al. 2021), all of which affect orchids in Massachusetts (Frost 2023). Other specific threats include changes in climate, which might cause a disassociation with its pollinators.
Conservation
This is a green flowering orchid that might typically grow in a lush understory making discovery of populations a bit more challenging than other species. Given the apparent sharp decline in frog orchid, much more de novo surveying is needed as well as more thorough resurvey work on known populations. All populations need increased monitoring.
Management needs include removal and control of any invasive species, as well as avoidance of timber harvest in the area.
Research needs include light management to answer the question as to whether this species might respond to increases in light reaching the forest floor. Many other research needs are those that would specifically address the threats listed above.
References
Anthony, M. A., S. D. Frey, and K. A. Stinson. 2017. Fungal community homogenization, shift in dominant trophic guild, and appearance of novel taxa with biotic invasion. Ecosphere 8: n/a-n/a.
Bertin, R. I., M. G. Hickler, K. B. Searcy, G. Motzkin, and P. P. Grima. 2020. Vascular Flora of Franklin County, Massachusetts. New England Botanical Club. 390 pp.
Bertin, R. I, Rawinski, T. J. (2012) Vascular Flora of Worcester County, Massachusetts. NewEngland Botanical Club. 291pp.
Brumback, W. E., S. Cairns, M. B. Sperduto, and C. W. Fyler. 2011. Response of an Isotria medeoloides Population to Canopy Thinning. Northeastern Naturalist 18: 185–196.
Consortium of Northeastern Herbaria. 2025. Herbarium records. https://portal.neherbaria.org/portal/collections/list.php. Accessed 3/13/2025.
Figura, T., M. Weiser, and J. Ponert. 2020. Orchid seed sensitivity to nitrate reflects habitat preferences and soil nitrate content. Plant Biology 22: 21–29.
Frost, Karro. 2023. Species listing proposal for Platanthera hookeri. Massachusetts Natural Heritage & Endangered Species. Massachusetts Division of Fisheries and Wildlife, Westborough, MA.
Gleason, H.A. and A. Cronquist. 1991. Manual of Vascular Plants of Northeastern United States and Adjacent Canada, Second Edition. The New York Botanical Garden, Bronx, New York.
Haines, A. 2011. Flora Novae Angliae. The New England Wild Flower Society. Yale University Press, New Haven, CT.
iNaturalist 2025. Available from https://www.inaturalist.org. Accessed 24 March 2025
Jenkins, J. C., Motzkin, G., Ward, K. 2008. The Harvard Forest Flora. An Inventory, Analysis and Ecological History. Harvard Forest, Harvard University, Petersham, Massachusetts. Harvard Forest PaperNo.28.
Knapp WM, Wiegand R (2014) orchid (orchidaceae) decline in the Catoctin Mountains, Frederick County, Maryland as documented by a long-term dataset. Biodivers Conserv 23:1965–1976. https://doi.org/10.1007/s10531-014-0698-2
McCormick, M. K., K. L. Parker, K. Szlavecz, and D. F. Whigham. 2013. Native and exotic earthworms affect orchid seed loss. AoB PLANTS 5: plt018.
NatureServe. 2025. NatureServe Network Biodiversity Location Data accessed through NatureServe Explorer [web application]. NatureServe, Arlington, Virginia. Available https://explorer.natureserve.org/. Accessed: 3/19/2025
Reddoch, J.M., and A.H. Reddoch. 2007. Population Ecology of Platanthera hookeri (orchidaceae) in Southwestern Quebec, Canada. Journal of the Torrey Botanical Society 134(3): 369-378.
Searcy, K. B. 2008. Vascular Flora of the Greater Mount Holyoke Range, Hampshire County, Massachusetts. Special Publication of the New England Botanical Club.
Sheviak, C. J. 1990. Biological considerations in the management of temperate terrestrial orchid habitats. New York State Museum Bulletin 471: 194–196.
Sheviak, C.J. 2003. Platanthera in Flora of North America. Editorial Committee, editors. Flora of North America, Volume 26 Page 555. Oxford University Press, New York, New York.Smith, Welby R.. 2012. Native orchids of Minnesota [revision of orchids of Minnesota by Welby R. Smith]. University of Minnesota Press, Minneapolis, London. xxxi + 254 pp. Illustrated by Vera Ming Wong and Bobby Angell. ISBN 978-0-8166-7823-5. Paperback.
Smith, Welby R.. 2012. Native orchids of Minnesota [revision of orchids of Minnesota by Welby R. Smith]. University of Minnesota Press, Minneapolis, London. xxxi + 254 pp. Illustrated by Vera Ming Wong and Bobby Angell. ISBN 978-0-8166-7823-5. Paperback.
Staudinger, M.D., A.V. Karmalkar, K. Terwilliger, K. Burgio, A. Lubeck, H. Higgins, T. Rice, T.L. Morelli, A. D'Amato. 2024. A regional synthesis of climate data to inform the 2025 State Wildlife Action Plans in the Northeast U.S. DOI Northeast Climate Adaptation Science Center Cooperator Report. 406 p. https://doi.org/10.21429/t352-9q86
Whalen, E. D., R. G. Smith, A. S. Grandy, and S. D. Frey. 2018. Manganese limitation as a mechanism for reduced decomposition in soils under atmospheric nitrogen deposition. Soil Biology and Biochemistry 127: 252–263.
Whigham, D., M. McCormick, H. Brooks, B. Josey, R. Floyd, and J. Applegate. 2021. Isotria medeoloides, a North American Threatened orchid: Fungal Abundance May Be as Important as Light in Species Management. Plants 10: 1924.
Contact
| Date published: | May 6, 2025 |
|---|